406457
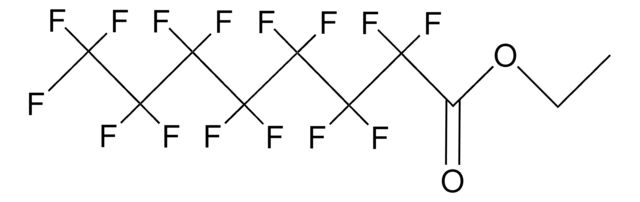
Methyl perfluorooctanoate
98%
Synonym(s):
Methyl pentadecafluorooctanoate
About This Item
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.305 (lit.)
bp
159-160 °C (lit.)
density
1.786 g/mL at 25 °C (lit.)
functional group
ester
fluoro
SMILES string
COC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C9H3F15O2/c1-26-2(25)3(10,11)4(12,13)5(14,15)6(16,17)7(18,19)8(20,21)9(22,23)24/h1H3
InChI key
XOCNYZFAMHDXJK-UHFFFAOYSA-N
General description
Application
- Synthesis of monodisperse perfluoroalkyl N-polyethoxylated amides, potential nonionic fluorinated surfactants.
- To investigate the mutual solubility curves of fluorocarbon-hydrocarbon systems.
- Synthesis of fluorine-containing monomer, N-(5-hydroxypent-1-yl)perfluorooctaneamide.
- To investigate the vinyl ether formulations for use in step and flash imprint lithography.
- Preparation of 2-chloro-1,1-dicyano-2-(perfluoroheptyl)ethylene, a precursor for synthesizing perfluorocarbon-soluble dyes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Lact. - Repr. 1B - STOT RE 1
Target Organs
Liver
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service