All Photos(3)
About This Item
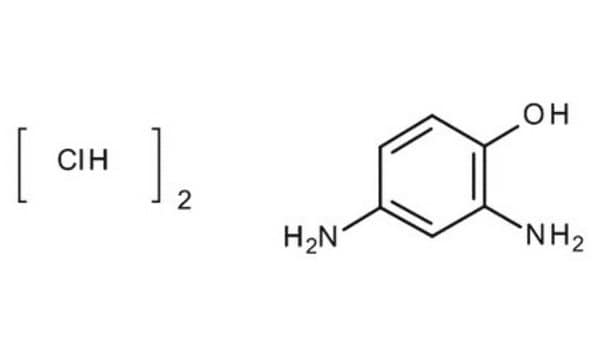
Linear Formula:
(H2N)2C6H3OH
CAS Number:
Molecular Weight:
124.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
161-165 °C (lit.)
SMILES string
Nc1cccc(O)c1N
InChI
1S/C6H8N2O/c7-4-2-1-3-5(9)6(4)8/h1-3,9H,7-8H2
InChI key
PCAXITAPTVOLGL-UHFFFAOYSA-N
Related Categories
General description
2,3-Diaminophenol is an aromatic diamine and forms Pd(II) and Pt(II) complexes. 2,3-Diaminophenol reacts with 2,4-pentanedione to yield the corresponding benzo[b][1,4]diazepinium salts. 2,3-Diaminophenol reacts with salicylaldehyde or 5-bromosalicylaldehyde in absolute ethanol to yield new unsymmetrical Schiff base.
Application
2,3-Diaminophenol was used:
- in one-pot microwave assisted synthesis of amino-1,5-benzoxazepines and hydroxyl-1,5-benzodiazepines
- in the electrosynthesis of poly(2,3-diaminophenol) via electro-oxidation
- in a synthesis of tetradentate Schiff base complexes via reaction with salicylaldehyde or 5-bromosalicylaldehyde and metals such as Mn(III), Ni(II) and Cu(II)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Constantinos G Neochoritis et al.
Journal of medicinal chemistry, 53(23), 8409-8420 (2010-11-06)
Amino-1,5-benzoxazepines 2 and 5 and hydroxyl-1,5-benzodiazepines 3 and 6 have been synthesized in one-pot solvent-free conditions from 2,3-diaminophenol and ketones through microwave assisted acid catalysis, the benzoxazepine/benzodiazepine ratio depending on the R(1) and R(3) aryl substituents. The otherwise inaccessible and
Electropolymerization of 2, 3-diaminophenol.
Del Valle MA, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 38(9), 1698-1703 (2000)
Transition Met. Chem. (London), 31, 169-169 (2006)
Unsymmetrical tetradentate Schiff base complexes derived from 2, 3-diaminophenol and salicylaldehyde or 5-bromosalicylaldehyde.
Ourari A, et al.
Transition Metal Chemistry, 31(2), 169-175 (2006)
M Pérez-Cabré et al.
Journal of inorganic biochemistry, 98(3), 510-521 (2004-02-28)
Pd(II) and Pt(II) new complexes with simple aromatic diamines were synthesised and characterised with the aim of studying their possible antitumour activity. The aromatic diamines chosen were 2,3-diaminotoluene (2,3 dat), 3,4-diaminotoluene (3,4 dat), 4,5-diaminoxylene (4,5 dax) and 2,3-diaminophenol (2,3 dap).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service