All Photos(1)
About This Item
Linear Formula:
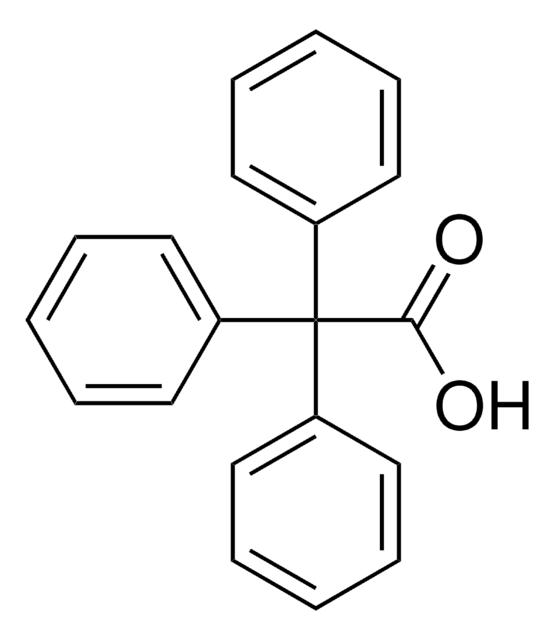
(C6H5)3CCH2CO2H
CAS Number:
Molecular Weight:
302.37
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
10.1 (vs air)
Assay
97%
mp
180-182 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)CC(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C21H18O2/c22-20(23)16-21(17-10-4-1-5-11-17,18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-15H,16H2,(H,22,23)
InChI key
XMSJLUKCGWQAHO-UHFFFAOYSA-N
Related Categories
General description
Reaction of 3,3,3-triphenylpropionic acid with lead tetraacetate in benzene, acetonitrile or chlorobenzene solution has been investigated.
Application
3,3,3-Triphenylpropionic acid has been used in the preparation of trityl-deprotected aminoalkoxysilane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spacing and site isolation of amine groups in 3-aminopropyl-grafted silica materials: The role of protecting groups.
Hicks JC, et al.
Chemistry of Materials, 18(21), 5022-5032 (2006)
Concurrent Carbon-to-Oxygen Rearrangement, Cyclization, and Decarboxylation in the Reaction of 3, 3, 3-Triarylpropionic Acids with Lead Tetraacetate.
Starnes WH.
Journal of the American Chemical Society, 86(24), 5603-5611 (1964)
Quentin Duez et al.
Polymers, 11(4) (2019-04-19)
Several families of polymers possessing various end-groups are characterized by ion mobility mass spectrometry (IMMS). A significant contribution of the end-groups to the ion collision cross section (CCS) is observed, although their role is neglected in current fitting models described
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service