122831
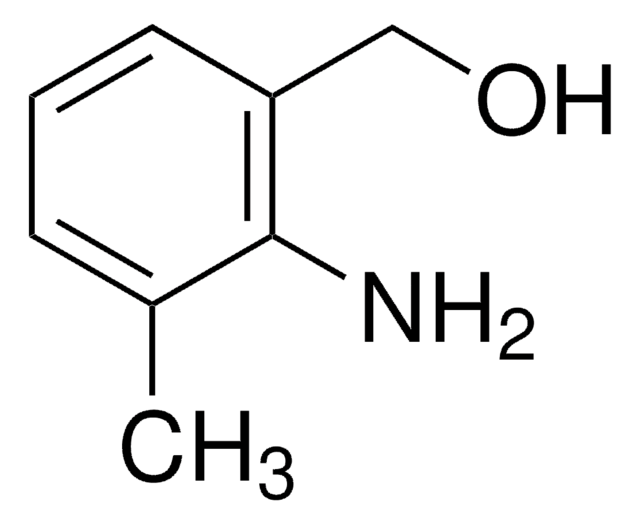
2-Aminobenzyl alcohol
98%
Synonym(s):
2-(Hydroxymethyl)aniline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4CH2OH
CAS Number:
Molecular Weight:
123.15
Beilstein:
1072211
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
162 °C/15 mmHg (lit.)
mp
81-83 °C (lit.)
SMILES string
Nc1ccccc1CO
InChI
1S/C7H9NO/c8-7-4-2-1-3-6(7)5-9/h1-4,9H,5,8H2
InChI key
VYFOAVADNIHPTR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Aminobenzyl alcohol is oxidatively cyclised with an array of ketones in dioxane at 80°C in the presence of a ruthenium catalyst and KOH to give corresponding quinolines. It undergoes oxidation catalyzed by heterotrimetallic RuMnMn species on the hydrotalcite surface in the presence of O2 to yield 2-aminobenzaldehyde.
Application
2-Aminobenzyl alcohol was used in the synthesis of ethyl 2-hydroxymethylcarbanilate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Sundaraganesan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 61(3), 377-385 (2004-12-08)
The Fourier transform Raman and Fourier transform infrared spectra of 2-aminobenzyl alcohol (2ABA) were recorded in the solid phase. Geometry optimizations were done with out any constraint and harmonic vibrational wave numbers and several thermodynamic parameters were calculated for the
J P Chism et al.
Chemical research in toxicology, 2(3), 150-156 (1989-05-01)
Previous results have suggested that key intermediates in the activation of 2-nitrotoluene and 2,6-dinitrotoluene are 2-aminobenzyl alcohol and 2-amino-6-nitrobenzyl alcohol, respectively. In order to determine the metabolic pathway(s) involved in the activation steps, calf thymus DNA and [14C]-2-aminobenzyl alcohol or
Ruthenium-catalysed oxidative cyclisation of 2-aminobenzyl alcohol with ketones: modified Friedlaender quinoline synthesis.
Cho CS, et al.
Chemical Communications (Cambridge, England), 24, 2576-2577 (2001)
Heterotrimetallic RuMnMn species on a hydrotalcite surface as highly efficient heterogeneous catalysts for liquid-phase oxidation of alcohols with molecular oxygen.
Kohki Ebitani et al.
Angewandte Chemie (International ed. in English), 44(22), 3423-3426 (2005-04-30)
Facile intramolecular nucleophilic attack by alkoxide ions on ethyl and p-nitrophenyl carbamates.
J E Hutchins et al.
Journal of the American Chemical Society, 95(11), 3786-3790 (1973-05-30)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service