All Photos(1)
About This Item
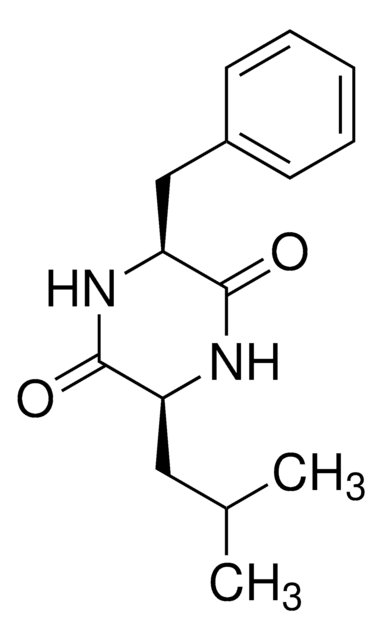
Empirical Formula (Hill Notation):
C12H16N2O3
CAS Number:
Molecular Weight:
236.27
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
Phe-Ala,
Assay
≥98.0% (TLC)
form
powder
color
white
storage temp.
−20°C
SMILES string
C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O
InChI
1S/C12H16N2O3/c1-8(12(16)17)14-11(15)10(13)7-9-5-3-2-4-6-9/h2-6,8,10H,7,13H2,1H3,(H,14,15)(H,16,17)/t8-,10-/m0/s1
InChI key
MIDZLCFIAINOQN-WPRPVWTQSA-N
Gene Information
human ... SLC15A1(6564)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jaeseung Kim et al.
The Journal of organic chemistry, 70(15), 5781-5789 (2005-07-16)
Four stereoisomers of a Phe-Ala silanediol dipeptide mimic have been evaluated as inhibitors of angiotensin-converting enzyme (ACE) and compared to ketone-based inhibitors reported by Almquist et al. One stereogenic center of the isomers was derived from the individual enantiomers of
D Meredith et al.
The American journal of physiology, 269(2 Pt 1), L137-L143 (1995-08-01)
The transport of a hydrolysis-resistant dipeptide, D-phenylalanyl-L-alanine (D-Phe-L-Ala), has been studied by high-performance liquid chromatography in rat lung epithelial cells and apical membrane vesicles. Time-dependent uptake of D-Phe-L-Ala into isolated type II pneumocytes was shown. Uptake was saturable, and Michaelis-Menten
U Wenzel et al.
Journal of cellular physiology, 178(3), 341-348 (1999-02-16)
Di- and tripeptides and peptide mimetics such as beta-lactam antibiotics are efficiently reabsorbed from the tubular lumen by a high-affinity peptide transporter. We have recently identified and characterized this H+-coupled high-affinity peptide transport system in the porcine proximal tubular cell
Jason Lamar et al.
Bioorganic & medicinal chemistry letters, 14(1), 239-243 (2003-12-20)
We describe herein the syntheses and evaluation of a series of C-termini pyridyl containing Phe*-Ala-based BACE inhibitors (5-19). In conjunction with four fixed residues at the P1 (Phe), P1' (Ala), P2' (Val), and P2' cap (Pyr.), rather detailed SAR modifications
Alessandro Sacchetti et al.
The Journal of organic chemistry, 76(3), 833-839 (2011-01-13)
The synthesis of a novel Phe-Ala dipeptide mimic, built up on a diazaspirocyclic lactam core, is presented. This new scaffold was evaluated for conformational mimicry of reverse turn by combining molecular modeling, IR, NMR, and X-ray diffraction experiments. All these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service