D98203
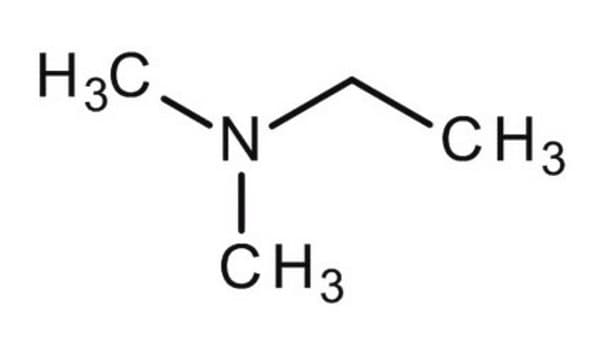
N,N-Diethylmethylamine
97%
Synonym(s):
N-Methyldiethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3N(C2H5)2
CAS Number:
Molecular Weight:
87.16
Beilstein:
1730930
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.389 (lit.)
bp
63-65 °C (lit.)
density
0.72 g/mL at 25 °C (lit.)
SMILES string
CCN(C)CC
InChI
1S/C5H13N/c1-4-6(3)5-2/h4-5H2,1-3H3
InChI key
GNVRJGIVDSQCOP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- N,N-Diethylmethylamine as lineshape standard for NMR above 130 K.: This study explores the use of N,N-Diethylmethylamine as a lineshape standard for nuclear magnetic resonance (NMR) spectroscopy at temperatures above 130 K, providing insights into its applications in high-precision NMR analysis (Fritzsching et al., 2018).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
-11.2 °F - closed cup
Flash Point(C)
-24 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ulderico Ulissi et al.
ChemSusChem, 11(1), 229-236 (2017-09-30)
The room-temperature molten salt mixture of N,N-diethyl-N-(2-methoxyethyl)-N-methylammonium bis(trifluoromethanesulfonyl) imide ([DEME][TFSI]) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) salt is herein reported as electrolyte for application in Li-O
P Roberts et al.
Biochemistry, 38(45), 14927-14940 (1999-11-11)
The steady-state reaction of trimethylamine dehydrogenase (TMADH) with the artificial electron acceptor ferricenium hexafluorophosphate (Fc(+)) has been studied by stopped-flow spectroscopy, with particular reference to the mechanism of inhibition by trimethylamine (TMA). Previous studies have suggested that the presence of
L Huang et al.
The Journal of biological chemistry, 271(23), 13401-13406 (1996-06-07)
The role played by the 6-S-cysteinyl-FMN bond of trimethylamine dehydrogenase in the reductive half-reaction of the enzyme has been studied by following the reaction of the slow substrate diethylmethylamine with a C30A mutant of the enzyme lacking the covalent flavin
Lihua Huang et al.
Analytical chemistry, 81(2), 567-577 (2008-12-17)
PEGylation of peptides and proteins presents significant challenges for structural characterization due to the heterogeneity of the poly(ethylene glycol) (PEG), the number of PEG moieties attached, and the site(s) of PEGylation. In this work, a novel and powerful methodology using
R J Rohlfs et al.
The Journal of biological chemistry, 269(49), 30869-30879 (1994-12-09)
The reductive half-reaction of trimethylamine dehydrogenase has been studied using the substrate diethylmethylamine over the pH range 6-10. It is found that the reaction occurs with three distinct and, under most conditions, fully resolved kinetic phases. The hyperbolic substrate concentration
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service