D39169
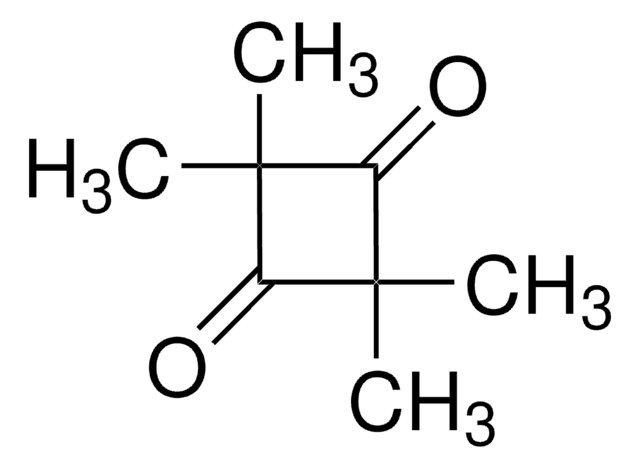
1,4-Dibromo-2,3-butanedione
99%
Synonym(s):
1,4-Dibromodiacetyl
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrCH2COCOCH2Br
CAS Number:
Molecular Weight:
243.88
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
117-119 °C (lit.)
SMILES string
BrCC(=O)C(=O)CBr
InChI
1S/C4H4Br2O2/c5-1-3(7)4(8)2-6/h1-2H2
InChI key
RZMOICJDRADLCT-UHFFFAOYSA-N
Application
- Catalyst-Free Multicomponent Cyclopolymerizations: A novel approach utilizing 1,4-Dibromo-2,3-butanedione in catalyst-free multicomponent cyclopolymerizations to synthesize functional polyiminofurans containing bromomethyl groups was developed. This method enhances the utility of 1,4-Dibromo-2,3-butanedione in producing advanced materials with potential applications in chemical and pharmaceutical industries (Zhu et al., 2021).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S H Vollmer et al.
Protein science : a publication of the Protein Society, 1(5), 678-687 (1992-05-01)
The bifunctional reagent 1,4-dibromobutanedione (DBBD) reacts covalently with pyruvate kinase from rabbit muscle to cause inactivation of the enzyme at a rate that is linearly dependent on the reagent concentration, giving a second order rate constant of 444 min-1 M-1.
Chui-Shan Tsang et al.
Chemical communications (Cambridge, England), (15)(15), 1999-2001 (2009-04-01)
Reaction of a pinene-based pyridylthioamide with 1,4-dibromo-2,3-butanedione in refluxing methanol yielded a new chiral pyridylthiazole ligand L which forms a dinuclear double-stranded helicate with Cu(i) ions; this helicate has opposite helical chirality when compared with its quaterpyridine analogue.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service