647292
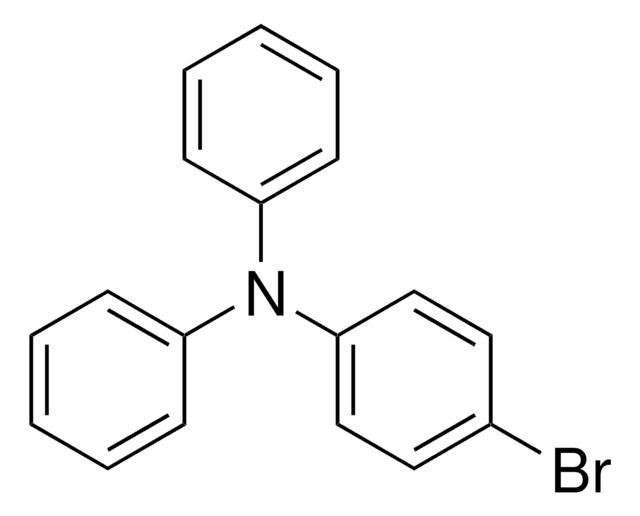
4-(Diphenylamino)phenylboronic acid
≥95%
Synonym(s):
4-(N,N-Diphenylamino)-1-phenylboronic acid, 4-(N,N-Diphenylamino)phenylboronic acid, 4-(N-Diphenylamino)phenylboronic acid, 4-(Diphenylamino)benzeneboronic acid, Triphenylamine-4-boronic acid
About This Item
Recommended Products
Assay
≥95%
form
powder
mp
110-115 °C (lit.)
SMILES string
OB(O)c1ccc(cc1)N(c2ccccc2)c3ccccc3
InChI
1S/C18H16BNO2/c21-19(22)15-11-13-18(14-12-15)20(16-7-3-1-4-8-16)17-9-5-2-6-10-17/h1-14,21-22H
InChI key
TWWQCBRELPOMER-UHFFFAOYSA-N
Related Categories
Application
- Strong multiphoton-excited blue photoluminescence and lasing from ladder-type oligo(p-phenylene)s
- Suzuki coupling reactions
- Ligand-free Suzuki reaction
Reagent used in Preparation of
- Push-pull arylvinyldiazine chromophores
- Benzothiadiazole-based fluorophores contg. triphenylamine functionality
- Blue light-emitting and hole-transporting materials for electroluminescent devices
- p-quaterphenyls laterally substituted with dimesitylboryl group for use as solid-state blue emitters
- Efficient sensitizers for dye-sensitized solar cells
- Prange electroluminescent materials for single-layer white polymer OLEDs
- Eeep-blue organic light emitting devices (OLEDs)
- Ligands for Organic Photovoltaic cells
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Self-healing soft electronic materials offer potential cost savings and reduced electronic waste.
Self-healing soft electronic materials offer potential cost savings and reduced electronic waste.
Self-healing soft electronic materials offer potential cost savings and reduced electronic waste.
Self-healing soft electronic materials offer potential cost savings and reduced electronic waste.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![B-[4-(1,2,2-Triphenylethenyl)phenyl]boronic acid](/deepweb/assets/sigmaaldrich/product/structures/121/044/864e0829-e1de-4170-aae4-16c2b3ce4111/640/864e0829-e1de-4170-aae4-16c2b3ce4111.png)





![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)