All Photos(1)
About This Item
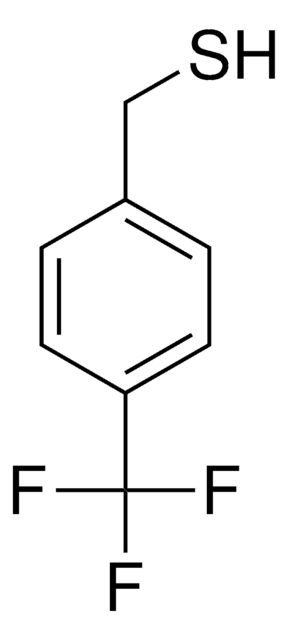
Linear Formula:
FC6H4CH2SH
CAS Number:
Molecular Weight:
142.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.545 (lit.)
bp
72-74 °C/15 mmHg (lit.)
density
1.157 g/mL at 25 °C (lit.)
functional group
fluoro
thiol
SMILES string
Fc1ccc(CS)cc1
InChI
1S/C7H7FS/c8-7-3-1-6(5-9)2-4-7/h1-4,9H,5H2
InChI key
RKTRHMNWVZRZJQ-UHFFFAOYSA-N
General description
4-Fluorobenzyl mercaptan undergoes reaction with p-chloranil to afford mainly 2,5-dichloro-3,6-S-disubstituted benzoquinone and 2,6-dichloro-3,5-S-disubstituted benzoquinone.
Application
4-Fluorobenzyl mercaptan may be used in the synthesis of the degradation products of fluorapacin, namely bis(4-fluorobenzyl)disulfide and bis(4-fluorobenzyl)tetrasulfide.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
170.0 °F - closed cup
Flash Point(C)
76.67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The synthesis of new cyclic thioquinone derivatives.
Ibis C and Ozsoy-Gunes Z.
Heteroatom Chem., 21(6), 446-452 (2010)
Yimei Bao et al.
Journal of pharmaceutical and biomedical analysis, 48(3), 664-671 (2008-08-06)
Bis(4-fluorobenzyl)trisulfide, fluorapacin, has been extensively developed as a promising new anticancer drug candidate. Its degradation products were identified and verified by the newly synthesized compounds bis(4-fluorobenzyl)disulfide (A) and bis(4-fluorobenzyl)tetrasulfide (B) which were resulted from the disproportionation of fluorapacin under forced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service