51030
Guanine hydrochloride
≥99.0%
Synonym(s):
2-Amino-6-hydroxypurine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H5N5O · HCl
CAS Number:
Molecular Weight:
187.59
Beilstein:
4159901
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0%
form
powder
ign. residue
≤0.05%
loss
≤3.0% loss on drying
mp
≥300 °C
storage temp.
2-8°C
SMILES string
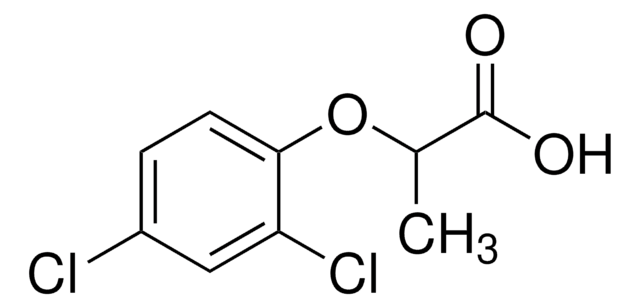
Cl.NC1=Nc2[nH]cnc2C(=O)N1
InChI
1S/C5H5N5O.ClH/c6-5-9-3-2(4(11)10-5)7-1-8-3;/h1H,(H4,6,7,8,9,10,11);1H
InChI key
IBAOFQIOOBQLHE-UHFFFAOYSA-N
Related Categories
General description
Guanine hydrochloride is the hydrochloride salt of the purine base, guanine. It is a component of basal medium.
Application
Guanine hydrochloride may be used in the synthesis of Na10[Pt2(μ-PO4)4(C5H3N5O)2].22H2O.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dhanashree Selvan et al.
ACS catalysis, 9(7), 5847-5859 (2019-07-26)
We report the construction of an artificial hydrogenase (ArH) by reengineering a Cu storage protein (Cspl) into a Ni-binding protein (NBP) employing rational metalloprotein design. The hypothesis driven design approach involved deleting existing Cu sites of Csp1 and identification of
Synthesis and X-ray structure of Na10[Pt2(?-PO4)4(C5H3N5O)2].22H2O, a complex with doubly deprotonated guanine anions coordinated to diplatinum(III).
El-Mehdawi R, et al.
Inorganic Chemistry, 25(20), 3714-3716 (1986)
The estimation of guanine and xanthine.
Hitchings GH.
The Journal of Biological Chemistry, 139(2), 843-854 (1941)
Srikrishna Pramanik et al.
Physical chemistry chemical physics : PCCP, 20(31), 20476-20488 (2018-07-26)
The development of base pair selective fluorescent binding probes and their interaction mode with nucleic acids have created great interest for sensing and biomedical applications. Herein, we have used chicken egg shell membrane (ESM) as a cost effective easily available
A factor required for the growth of Leuconostoc citrovorum.
H E SAUBERLICH et al.
The Journal of biological chemistry, 176(1), 165-173 (1948-10-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service