389137
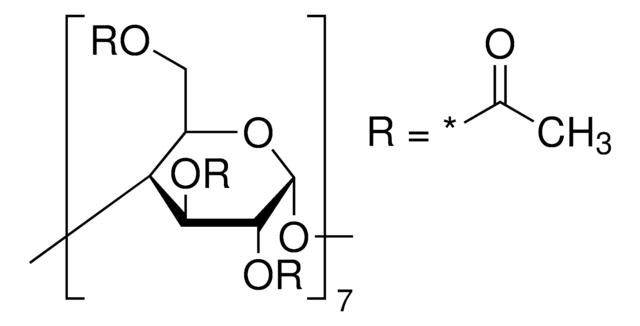
(2-Hydroxyethyl)-β-cyclodextrin
extent of labeling: ~0.7 mol per mol cellulose
Synonym(s):
2-HE-β-CD, 2-hydroxyethyl-β-CD, HP-β-CD
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
powder
Quality Level
optical activity
[α]20/D 120 to 140°, c = 1 in H2O
extent of labeling
~0.7 mol per mol cellulose
mp
260 °C (dec.) (lit.)
Looking for similar products? Visit Product Comparison Guide
General description
(2-Hydroxyethyl)-β-cyclodextrin is a chemically modified, hydroxyalkylated derivative of cyclodextrin that finds potential use mainly in cosmetics application.
Application
(2-Hydroxyethyl)-β-cyclodextrin may be used as a chiral selector to resolve cathinone derivatives, lactic acid and oxybutynin enantiomers by capillary electrophoresis (CE), and high performance liquid chromatography (HPLC) techniques.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclodextrin Fundamentals, Reactivity and Analysis
Environmental Chemistry for a Sustainable World (2018)
L Saavedra et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 766(2), 235-242 (2002-02-05)
The optimization of the separation conditions of the two optical isomers of lactic acid by a factorial design is reported. Initially, different chiral selectors were systematically investigated and then a experimental design with three quantitative factors (cyclodextrin concentration and background
Paweł Mateusz Nowak et al.
Electrophoresis, 39(19), 2406-2409 (2018-07-13)
Methcathinone (ephedrone), 4-methylmethcathinone (mephedrone), and 3-methylmethcathinone (metaphedrone) are toxicologically-important cathinone derivatives used commonly as designer drugs. In this work we show the first method allowing to separate simultaneously all these molecules in a chiral medium, ensuring good resolution between all
Design of Nanostructures for Versatile Therapeutic Applications
Pharmaceutical Nanotechnology (2018)
Equilibrium studies on enantioselective extraction of oxybutynin enantiomers by hydrophilic beta-cyclodextrin derivatives
Tang K, et al.
AIChE Journal, 57(11), 3027-3036 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service