286338
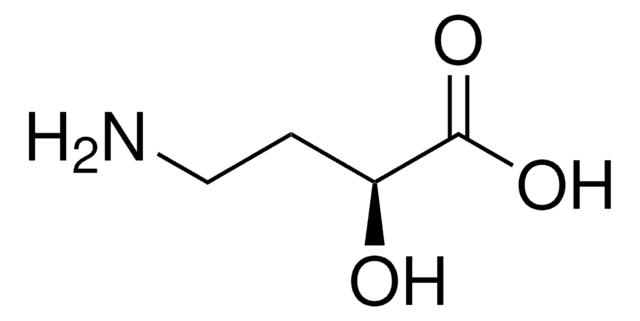
DL-Isoserine
98%
Synonym(s):
(±)-3-Amino-2-hydroxypropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CH(OH)CO2H
CAS Number:
Molecular Weight:
105.09
Beilstein:
1721413
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
235 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NCC(O)C(O)=O
InChI
1S/C3H7NO3/c4-1-2(5)3(6)7/h2,5H,1,4H2,(H,6,7)
InChI key
BMYNFMYTOJXKLE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B S Coller et al.
The Journal of biological chemistry, 268(28), 20741-20743 (1993-10-05)
Peptides containing sequences derived from the new NH2 terminus of the seven-transmembrane domain thrombin receptor after thrombin cleavage can activate platelets directly. We recently demonstrated that such peptides are readily cleaved and inactivated by plasma, serum, and endothelial cell-associated aminopeptidase
Nagarjuna Palyam et al.
The Journal of organic chemistry, 74(11), 4390-4392 (2009-05-02)
We report a new protocol for synthesis of L-1-deoxymannojirimycin, L-1-deoxyidonojirimycin, and the N-isopropyl derivative of the latter compound from the readily available precursors (S)-isoserinal hydrate and 2-tert-butyl-2-methyl-1,3-dioxan-5-one. The key steps include diastereoselective proline-catalyzed syn aldol transformation and a reductive amination/cyclization.
Thomas Rühl et al.
Chemical communications (Cambridge, England), (15)(15), 1630-1631 (2002-08-13)
The photolytic decomposition of trifunctional carbene generating photoaffinity probes in methanolic solution was studied, a cleavage reaction with butylamine in water, the conjugation with a ligand (moenomycin), and experiments that demonstrate that the fully armed probes interact with penicillin-binding protein
J Du et al.
Nucleosides & nucleotides, 17(1-3), 1-13 (1998-08-26)
Asymmetric synthesis of N-substituted oxazolidinyl nucleosides has been accomplished from L-isoserine, trans- and cis-Oxazolidine intermediates (4 and 5) were stereoselectively constructed from N-protected L-isoserine with a menthoxycarbonyl group by the condensation with benzoyloxy acetaldehyde dimethyl acetal in a ratio of
Microbial resolution of 2-hydroxy-3-nitropropionic acid for synthesis of optically active isoserine.
Y Yasohara et al.
Bioscience, biotechnology, and biochemistry, 65(5), 1258-1260 (2001-07-07)
The biocatalytic stereoselective hydrolysis of 2-hydroxy-3-nitropropionic acid esters was studied. Forty enzymes and three hundred microorganism strains were examined for their ability to hydrolyze ethyl 2-hydroxy-3-nitropropionic acid. Nocardia globerula IFO13150 gave n-butyl (R)-2-hydroxy-3-nitropropionate with a 92% enantiomeric excess (ee) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service