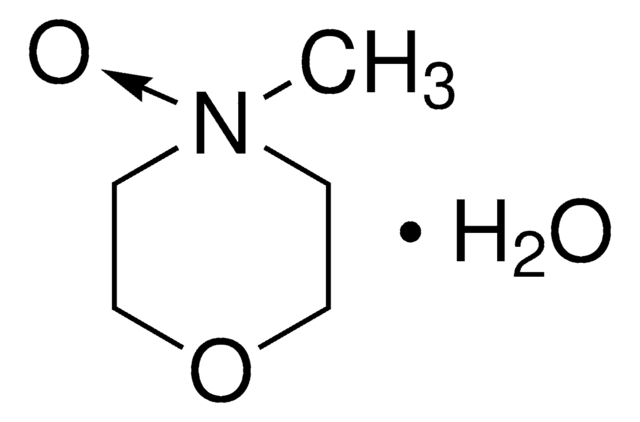
258822
4-Methylmorpholine N-oxide solution
50 wt. % in H2O
Synonym(s):
NMMO solution, NMO solution, NSC 73198, NSC 82153
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H11NO2
CAS Number:
Molecular Weight:
117.15
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: oxidant
concentration
50 wt. % in H2O
refractive index
n20/D 1.4201
pH
9.00 ( in neat)
bp
118.5 °C
mp
−20 °C
density
1.13 g/mL at 25 °C
functional group
ether
storage temp.
2-8°C
SMILES string
C[N+]1([O-])CCOCC1
InChI
1S/C5H11NO2/c1-6(7)2-4-8-5-3-6/h2-5H2,1H3
InChI key
LFTLOKWAGJYHHR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 4-Methylmorpholine N-oxide (NMO) is widely used as a co-oxidant to regenerate osmium tetroxide (OsO4) catalyst during dihydroxylation of alkenes.
- In the presence of catalytic amounts of tetra-n-propylammonium perruthenate (TPAP), NMO oxidizes secondary amines to the corresponding imines.
- 4-Methylmorpholine N-oxide solution can be used to oxidize activated primary halides to aldehydes and secondary halides to ketones, respectively.
- It can also be used to promote stereoselective intermolecular Pauson-Khand reaction for the synthesis of cyclopentenones.
Oxidant for synthesis of novel organic polymer - inorganic hybrid for use as a catalyst for asymmetrical epoxidation
Reagent or Reactant for:
Used as a pretreatment for techno-economical study of ethanol and biogas production from spruce wood
Reagent or Reactant for:
- Cyclocondensation and cyclization in the enantioselective synthesis of oxazolomycin A
- Wharton rearrangement and stereoselective dihydroxylation reactions
- Reduction of amine N-oxides by diboron reagents
- Synthesis of polysaccharide blend fibers
- Synthesis of cellulose / modified nano-SiO2 composite packaging films
- Copper-catalyzed oxidative coupling for preparation of propargylamines
Used as a pretreatment for techno-economical study of ethanol and biogas production from spruce wood
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asymmetric dihydroxylation via ligand-accelerated catalysis.
Jacobsen E N, et al.
Journal of the American Chemical Society, 110(6), 1968-1970 (1988)
Oxidation of activated halides to aldehydes and ketones by N-methylmorpholine-N-oxide.
Griffith W P, et al.
Synthetic Communications, 22(13), 1967-1971 (1992)
Catalytic Osmium Tetroxide Oxidation of Olefins: cis?1, 2?Cyclohexanediol.
VanRheenen V, et al.
Organic Syntheses, 43-43 (1988)
Catalytic oxidation of secondary amines with tetra-n-propylammonium perruthenate.
Goti A and Romani M
Tetrahedron Letters, 35(35), 6567-6570 (1994)
Patrik R Lennartsson et al.
Bioresource technology, 102(6), 4425-4432 (2011-01-21)
A complete process for the production of bioethanol and fungal biomass from spruce and birch was investigated. The process included milling, pretreatment with N-methylmorpholine-N-oxide (NMMO), washing of the pretreated wood, enzymatic hydrolysis, and cultivation of the zygomycetes fungi Mucor indicus.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service