188220
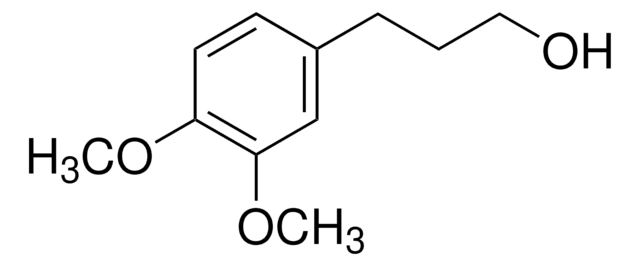
5-Phenyl-1-pentanol
98%
Synonym(s):
Benzenepentanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5(CH2)5OH
CAS Number:
Molecular Weight:
164.24
Beilstein:
1935233
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.516 (lit.)
bp
155 °C/20 mmHg (lit.)
density
0.975 g/mL at 25 °C (lit.)
SMILES string
OCCCCCc1ccccc1
InChI
1S/C11H16O/c12-10-6-2-5-9-11-7-3-1-4-8-11/h1,3-4,7-8,12H,2,5-6,9-10H2
InChI key
DPZMVZIQRMVBBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
5-Phenyl-1-pentanol undergoes oxidation in the presence of ceric ammonium nitrate to yield 2-benzyltetrahydrofuran.
Application
5-Phenyl-1-pentanol was used in the synthesis of:
- methoxy and fluorine analogs of N-(piperidinyl)-1-(2,4-dichlorophenyl)-4-methyl-5-(4-pentylphenyl)-1H-pyrazole-3-carboxamide (O-1302)
- 2-[5-(4-iodophenyl)-pentyl]oxirane-2-carboxylic acid ethyl ester
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H G Abbas et al.
International journal of radiation applications and instrumentation. Part A, Applied radiation and isotopes, 42(1), 7-11 (1991-01-01)
A method is described for the synthesis, purification and radiolabelling of [123I/131I]2-[5-(4-iodophenyl)-pentyl]oxirane-2-carboxylic acid ethyl ester. For the synthesis of this new agent, 5-phenylpentyl bromide (1), synthesized by reacting 5-phenyl-1-pentanol with sodium bromide under acidic conditions, was converted to diethyl 5-phenylpentylmalonate
Shintaro Tobiishi et al.
Chemical & pharmaceutical bulletin, 55(8), 1213-1217 (2007-08-02)
Methoxy and fluorine analogs substituted on the terminal carbon of the pentyl chain of N-(piperidinyl)-1-(2,4-dichlorophenyl)-4-methyl-5-(4-pentylphenyl)-1H-pyrazole-3-carboxamide (O-1302) were synthesized in a multi-step process from 5-phenyl-1-pentanol, which was based on the 1,5-diarylpyrazole core template of N-(piperidinyl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716) through condensation of the respective
Cyclic ether formation in oxidations of primary alcohols by cerium (IV). Reactions of 5-phenyl-1-pentanol, 4-phenyl-1-butanol, and 3-phenyl-1-propanol with ceric ammonium nitrate.
Doyle MP, et al.
The Journal of Organic Chemistry, 40(10), 1454-1456 (1975)
Hidetaka Nagatomo et al.
Bioscience, biotechnology, and biochemistry, 69(1), 128-136 (2005-01-25)
Hyperthermostable beta-glucosidase from Pyrococcus furiosus was enclosed in gelatin gel by cross-linking with transglutaminase. Gelatin-immobilized beta-glucosidase was considerably more thermostable than the native enzyme. Lyophilized immobilisate was stored at 90 degrees C for 1 month without loss of activity. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service