All Photos(1)
About This Item
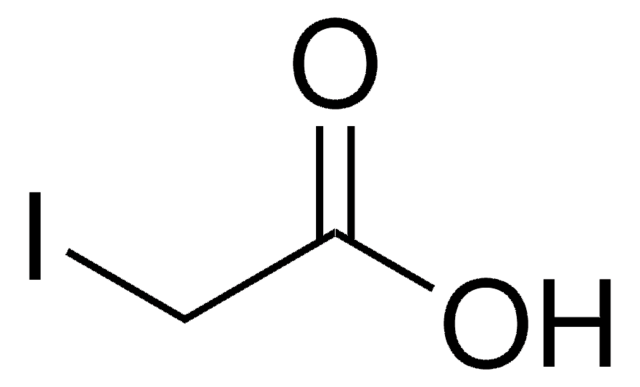
Empirical Formula (Hill Notation):
C5H5ClN2O2
CAS Number:
Molecular Weight:
160.56
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
257 °C (dec.) (lit.)
SMILES string
ClCC1=CC(=O)NC(=O)N1
InChI
1S/C5H5ClN2O2/c6-2-3-1-4(9)8-5(10)7-3/h1H,2H2,(H2,7,8,9,10)
InChI key
VCFXBAPEXBTNEA-UHFFFAOYSA-N
General description
6-(Chloromethyl)uracil on chlorination with sulfuryl chloride in acetic acid yields 5-chloro-6-(chloromethyl)uracil.
Application
6-(Chloromethyl)uracil was used in the synthesis of:
- 5-bromo-6-(chloromethyl)uracil
- pteridine compounds, potential anticancer agents
- substituted uracil pyridinium compounds, potential inhibitors of thymidine phosphorylase
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W Wang et al.
International journal of radiation biology, 71(4), 387-399 (1997-04-01)
In this work radicals generated by dissociative electron attachment to iodoacetamide (H2NCOCH2.) and 6-chloromethyluracil (U5CH2.) are suggested to react with DNA nucleotides in frozen aqueous solutions via either hydrogen abstraction or addition to the double bonds of the bases. Methyl
Prem M S Chauhan et al.
Bioorganic & medicinal chemistry, 13(10), 3513-3518 (2005-04-26)
Several pteridine analogues 4-13, 23-26 have been synthesized and tested in vitro against three cancer cell lines, MCF7 (breast), NCI-H460 (lung) and SF-268 (CNS). All tested pteridines can serve as novel templates for the anticancer chemotherapy and can serve as
Shingo Yano et al.
Bioorganic & medicinal chemistry, 12(13), 3431-3441 (2004-06-10)
A series of novel 6-methylene-bridged uracil derivatives have been prepared as inhibitors of human thymidine phosphorylase (TP). To enhance the in vivo antitumor activity of fluorinated pyrimidine 2'-deoxyribonucleosides such as 2'-deoxy-5-(trifluoromethyl)uridine (F(3)dThd), a potent TP inhibitor preventing their degradation to
J Klosa
Arzneimittel-Forschung, 30(2), 228-231 (1980-01-01)
The synthesis of new uracil derivatives is described. In 4-chloromethyluracil, chlorine can be easily exchanged under mild conditions for amine, aniline, hydrazine, and phenol.
Paul E Murray et al.
Bioorganic & medicinal chemistry, 10(3), 525-530 (2002-01-30)
A series of water soluble N(1)- and C(6)-substituted uracil pyridinium compounds were prepared as potential inhibitors of thymidine phosphorylase (TP). The C(6)-uracil substituted derivatives were the most active. 1-[(5-Chloro-2,4-dihydroxypyrimidin-6-yl)methyl]pyridinium chloride, was identified as the best inhibitor being 5-fold more potent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service