147834
Michler′s ketone
98%
Synonym(s):
4,4′-Bis(dimethylamino)benzophenone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
[(CH3)2NC6H4]2CO
CAS Number:
Molecular Weight:
268.35
Beilstein:
790733
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
powder
mp
174-176 °C (lit.)
SMILES string
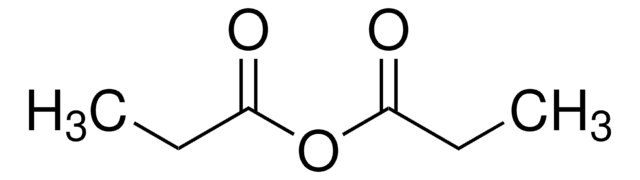
CN(C)c1ccc(cc1)C(=O)c2ccc(cc2)N(C)C
InChI
1S/C17H20N2O/c1-18(2)15-9-5-13(6-10-15)17(20)14-7-11-16(12-8-14)19(3)4/h5-12H,1-4H3
InChI key
VVBLNCFGVYUYGU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Michler′s ketone (MK) is a derivative of benzophenone that shows temperature dependent phosphorescence. It also exhibits triplet-triplet absorption spectra at a lower temperature.
Application
MK can be used as an additive that acts as a photoinitiator in the preparation of dyes. It can also be used as a precursor material in the synthesis of 4,4′-bis{N,N,dimethyl, N (2-ethoxy carbonyl-1-propenyl) ammonium hexafluoro antimonate}benzophenone (MKEA).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Eye Dam. 1 - Muta. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
L G Rushing et al.
Journal of chromatographic science, 18(5), 224-232 (1980-05-01)
Sensitive and specific procedures are described for the analysis of residues of gentian violet in animal feed, human urine, and wastewater at levels of 1000 ppm down to 10 ppb, 1 ppb, and 10 ppb, respectively. The cleaned-up extracts were
R F Struck et al.
Xenobiotica; the fate of foreign compounds in biological systems, 11(8), 569-567 (1981-08-01)
1. Studies on the metabolism of 14C-Michler's ketone (4,4'-bis-(dimethylamino)[carbonyl- 14C]benzophenone) in rats have revealed that this carcinogen is subject to demethylation, ring-hydroxylation and N-acetylation after adjacent methyl groups have been removed. 2 As identified by mass spectral analysis, microsomal metabolites
Cinzia Chiappe et al.
The journal of physical chemistry. A, 110(14), 4937-4941 (2006-04-08)
Michler's ketone (MK) and tetracyanoethene (TCNE) may be used as a UV-vis probe to investigate the solvent properties of ionic liquids (ILs). In molecular solvents, MK and TCNE give an electron donor-acceptor (EDA) complex, a zwitterionic species or a radical
H B Li et al.
Journal of analytical toxicology, 24(8), 704-707 (2000-12-08)
Methylmercury is the most toxic among the mercury species. In order to provide a quick method to screen samples for methylmercury, a highly sensitive spectrophotometric method was established. The method was based on formation of the red complex of methylmercury
L Castle et al.
Food additives and contaminants, 14(1), 45-52 (1997-01-01)
A survey of retail samples was conducted in two phases with 50 general paper and board food contact materials and articles analysed in 1992, and 121 samples, specifically of printed cartonboard, analysed in 1995. Packaging samples were extracted with ethanol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service