All Photos(1)
About This Item
Empirical Formula (Hill Notation):
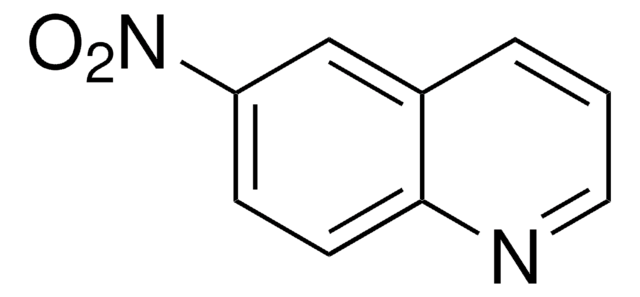
C9H7N3O2
CAS Number:
Molecular Weight:
189.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
272-273 °C (dec.) (lit.)
functional group
nitro
SMILES string
Nc1c(ccc2ncccc12)[N+]([O-])=O
InChI
1S/C9H7N3O2/c10-9-6-2-1-5-11-7(6)3-4-8(9)12(13)14/h1-5H,10H2
InChI key
TYBYHEXFKFLRFT-UHFFFAOYSA-N
General description
Electrochemical reduction of 5-amino-6-nitroquinoline has been studied at carbon paste electrode by differential pulse voltammetry, direct current voltammetry, adsorptive stripping voltammetry and HPLC with electrochemical detection.
Application
5-Amino-6-nitroquinoline was used in preparation of imidazoquinoline derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Determination of 5-amino-6-nitroquinoline at a carbon paste electrode.
Nemcova L, et al.
Collection of Czechoslovak Chemical Communications, 74(10), 1477-1488 (2009)
J H Weisburger et al.
Environmental health perspectives, 67, 121-127 (1986-08-01)
Because mutagens typified by 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) observed in cooked foods are widely consumed, detailed studies of their biochemical and biological properties including carcinogenicity are most important. IQ induces unscheduled DNA synthesis in liver cells, which when taken together with its
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service