104051
Picolinamide
98%
Synonym(s):
2-Pyridinecarboxamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O
CAS Number:
Molecular Weight:
122.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
110 °C (dec.) (lit.)
functional group
amide
SMILES string
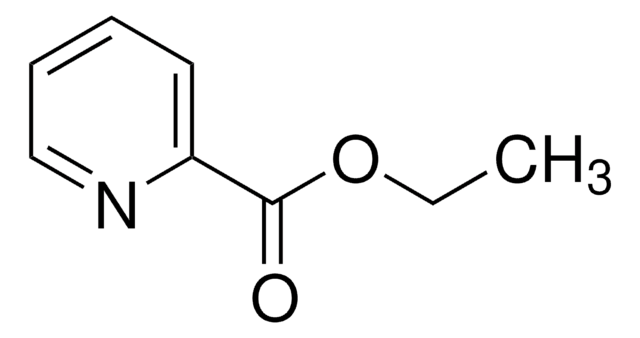
NC(=O)c1ccccn1
InChI
1S/C6H6N2O/c7-6(9)5-3-1-2-4-8-5/h1-4H,(H2,7,9)
InChI key
IBBMAWULFFBRKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Picolinamide was used as template in preparation of molecular imprinting polymer. Picolinamide was used in a study to evaluate kinetics and mechanism of liberation of picolinamide from chromium(III)-picolinamide complexes in HClO4.
Biochem/physiol Actions
Picolinamide is potential inhibitor of poly (ADP-ribose) synthetase of nuclei from rat pancreatic islet cells. Picolinamide acts as bidentate ligand and forms complexes with lanthanide nitrates, thiocyanates and perchlorates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bambang Kuswandi et al.
Analytica chimica acta, 591(2), 208-213 (2007-05-08)
A disposable ion-selective optode for mercury based on trityl-picolinamide (T-Pico) as neutral ionophore was developed. The sensing layer consist of plasticised PVC incorporating T-Pico as a selective ionophore for Hg2+, ETH 5418 as a chromoionophore, and potassium tetrakis[3,5-bis(trifluoromethyl)phenyl] borate as
Jeremy L Yap et al.
Organic & biomolecular chemistry, 10(15), 2928-2933 (2012-03-08)
By conducting a structure-activity relationship study of the backbone of a series of oligoamide-foldamer-based α-helix mimetics of the Bak BH3 helix, we have identified especially potent inhibitors of Bcl-x(L). The most potent compound has a K(i) value of 94 nM
Manuela Jörg et al.
ChemMedChem, 16(1), 216-233 (2020-08-28)
This study investigated the structure-activity relationships of 4-phenylpyridin-2-one and 6-phenylpyrimidin-4-one M1 muscarinic acetylcholine receptor (M1 mAChRs) positive allosteric modulators (PAMs). The presented series focuses on modifications to the core and top motif of the reported leads, MIPS1650 (1) and MIPS1780
Mark Turlington et al.
Journal of medicinal chemistry, 56(20), 7976-7996 (2013-09-21)
Positive allosteric modulators (PAMs) of metabotropic glutamate receptor 5 (mGlu5) represent a promising therapeutic strategy for the treatment of schizophrenia. Both allosteric agonism and high glutamate fold-shift have been implicated in the neurotoxic profile of some mGlu5 PAMs; however, these
Ángel Manu Martínez et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(48), 11669-11676 (2017-06-22)
A practical picolinamide-directed C-H functionalization/alkyne annulation of benzylamine derivatives enabling access to the previously elusive 1,4-dihydroisoquinoline skeleton was developed using molecular O
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service