L8753
Leu-Pro hydrochloride
≥98%, suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
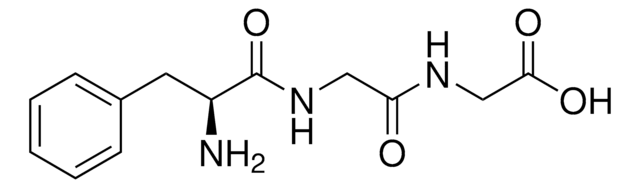
C11H20N2O3 · HCl
CAS Number:
Molecular Weight:
264.75
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
Leu-Pro hydrochloride,
Assay
≥98%
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
Cl.CC(C)CC(N)C(=O)N1CCCC1C(O)=O
InChI
1S/C11H20N2O3.ClH/c1-7(2)6-8(12)10(14)13-5-3-4-9(13)11(15)16;/h7-9H,3-6,12H2,1-2H3,(H,15,16);1H
InChI key
GZEPUBCBTCSTKP-UHFFFAOYSA-N
Biochem/physiol Actions
L-leucyl-L-proline (Leu-Pro) and its retro variant Pro-Leu may be used along with other proline dipeptides to study imprinting and chemotaxis in tetrahymena.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fengxia Zhang et al.
Molecular bioSystems, 6(5), 852-861 (2010-06-23)
A novel metabonomic method based on fast liquid chromatography coupled with ion trap-time of flight mass spectrometry (UFLC/MS-IT-TOF) was applied to study the metabolic changes of plasma and urine in depression and excess fatigue rats. Principal component analysis (PCA) and
G Csaba et al.
Bioscience reports, 17(6), 537-542 (1998-04-30)
Proline-glycine, proline-leucine and proline-valine dipeptides and their retro variants were used in the experiments to study the effects of pretreatment (imprinting) in Tetrahymena, by investigating fluorescein isothiocyanate (FITC)-conjugated peptide binding. The protozoan organism could differentiate between the proline-dipeptides containing different
L Köhidai et al.
Cell biology international, 21(6), 341-345 (1997-06-01)
Our investigations demonstrate that proline-containing dipeptides can provoke a chemosensory response from the unicellular Tetrahymena pyriformis. The chemotactic effects of the dipeptides have a close relationship with the side chain and the lipophilicity of the amino-terminal amino acid. Comparison of
G Schoetz et al.
Electrophoresis, 22(12), 2409-2415 (2001-08-25)
Dynamic capillary electrophoresis (DCE) and computer simulation of the elution profiles with the theoretical plate and the stochastic model has been applied to determine the isomerization barriers of the three dipeptides L-alanyl-L-proline, L-leucyl-L-proline, and L-phenylalanyl-L-proline. The separation of the rotational
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service