I8757
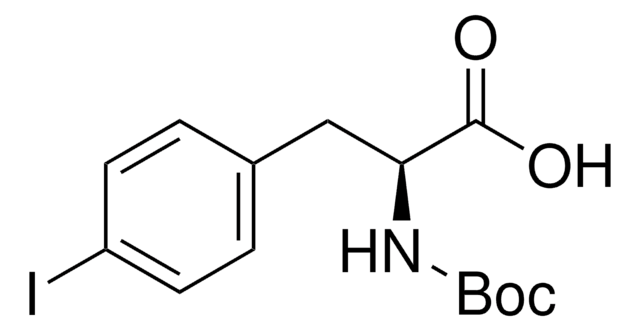
4-Iodo-L-phenylalanine
≥98.0% (TLC)
Synonym(s):
(S)-2-Amino-3-(4-iodophenyl)propanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H10INO2
CAS Number:
Molecular Weight:
291.09
Beilstein:
4411317
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
4-Iodo-L-phenylalanine,
Assay
≥98.0% (TLC)
form
powder
color
white to off-white
storage temp.
−20°C
SMILES string
N[C@@H](Cc1ccc(I)cc1)C(O)=O
InChI
1S/C9H10INO2/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)/t8-/m0/s1
InChI key
PZNQZSRPDOEBMS-QMMMGPOBSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
4-Iodo-L-phenylalanine may be used in protein engineering as a model unnatural α amino acid to alter primary amino acid composition via the opal (UGA) codon.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ulrich Hoffmanns et al.
Bioconjugate chemistry, 17(1), 204-213 (2006-01-19)
The Pd-catalyzed Sonogashira coupling of ferrocene alkyne derivatives as metal probes to iodophenylalanine containing peptides is described. 4-Iodophenylalanine was incorporated into dipeptides and the neuropeptide [Leu5]-enkephalin (Enk) by solid phase peptide synthesis, thereby creating a functional group suitable for the
Koichiro Kodama et al.
Journal of biochemistry, 148(2), 179-187 (2010-05-25)
A variety of unique codons have been employed to expand the genetic code. The use of the opal (UGA) codon is promising, but insufficient information is available about the UGA suppression approach, which facilitates the incorporation of non-natural amino acids
Ina Israel et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 66(4), 513-522 (2007-11-21)
This work describes the synthesis and the tumor affinity testing of no-carrier-added (n.c.a.) p-[(124)I]iodo-L-phenyalanine ([(124)I]IPA) and n.c.a. p-[(131)I]iodo-l-phenyalanine ([(131)I]IPA) as radiopharmaceuticals for imaging brain tumors with PET and for radionuclid-based therapy, respectively. Parameters for labeling were optimized with regard to
Veerle Kersemans et al.
European journal of nuclear medicine and molecular imaging, 33(8), 919-927 (2006-03-31)
In vitro in the R1M cell model and in vivo in the R1M tumour-bearing athymic model, both [(123)I]-2-iodo-L: -phenylalanine and [(123)I]-2-iodo-D: -phenylalanine have shown promising results as tumour diagnostic agents for SPECT. In order to compare these two amino acid
Dirk Hellwig et al.
European journal of nuclear medicine and molecular imaging, 35(1), 24-31 (2007-09-12)
Radioactive amino-acids accumulate in gliomas even with an intact blood-brain-barrier. L-3-[(123)I]-iodo-alpha-methyl-tyrosine (IMT) is well established for SPECT imaging of gliomas. Recently, we introduced p-[(123)I]-iodo-L-phenylalanine (IPA) for the characterisation of brain lesions. This study compares both tracers in glioma patients. Eleven
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service