Recommended Products
biological source
bovine (calf) thymus
type
Type III-S
form
powder
concentration
≥60% protein (biuret)
technique(s)
activity assay: suitable
solubility
H2O: soluble 10 mg/mL, clear to hazy, colorless to light yellow
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
Histone is suitable for use:
- as a positive control in the in vitro kinase assay to examine the ability of Cdc2-cyclin B1 to phosphorylate GST-CD-4 protein
- in Cdk5 activity assay
- in histone H1 kinase assay
- as a Poly(ADP-ribose) (PAR) acceptor in the assessment of in vitro PAR polymerase activity
- as an acceptor of 32P from [γ-32P]ATP in order to measure protein kinase activity of lysate from small and large luteal cells
Biochem/physiol Actions
Histones are a group of proteins characterized by high levels of lysine and arginine that form reversible complexes with DNA. Based on the fractions, the molar mass of histones varies between 11-21kDa. Five different fractions have been isolated and characterized. They include H1 (lysine rich), H2a and H2b that are slightly lysine rich, H3 and H4 that are arginine rich. H1 acts as a link between "beads" (nu bodies) on the chromatin chain.
Features and Benefits
Do not confuse Sigma "Type" designations with the "Fraction" designations used by Luck and co-workers.
Other Notes
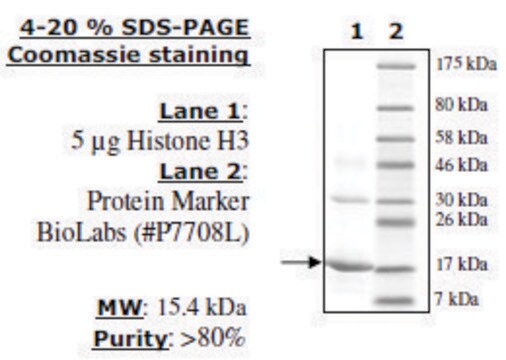
Characterized as mainly subgroup f1 by SDS-PAGE
Preparation Note
Lysine-rich fraction as isolated and described by de Nooij, E. and Westenbrink, H.G., Biochim. Biophys. Acta, 62, 608 (1962).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Balsas et al.
Journal of hematology & oncology, 10(1), 80-80 (2017-04-01)
Pharmacological inhibition of B cell receptor (BCR) signaling has recently emerged as an effective approach in a wide range of B lymphoid neoplasms. However, despite promising clinical activity of the first Bruton's kinase (Btk) and spleen tyrosine kinase (Syk) inhibitors
Charlotte Juillet et al.
Journal of medicinal chemistry, 64(2), 1197-1219 (2021-01-09)
Significant inhibition of Aurora B was achieved by the synthesis of simplified fragments of benzosceptrins and oroidin belonging to the marine pyrrole-2-aminoimidazoles metabolites isolated from sponges. Evaluation of kinase inhibition enabled the discovery of a synthetically accessible rigid acetylenic structural
Anne Cecília Nascimento da Cruz et al.
Pharmaceuticals (Basel, Switzerland), 12(4) (2019-11-21)
Fourteen arylsemicarbazone derivatives were synthesized and evaluated in order to find agents with potential anticancer activity. Cytotoxic screening was performed against K562, HL-60, MOLT-4, HEp-2, NCI-H292, HT-29 and MCF-7 tumor cell lines. Compounds 3c and 4a were active against the
Liang Zheng et al.
Haematologica, 105(4), 1107-1119 (2019-11-23)
Thrombotic thrombocytopenic purpura (TTP) is caused by severe deficiency of ADAMTS13 (A13), a plasma metalloprotease that cleaves endothelium-derived von Willebrand factor (VWF). However, severe A13 deficiency alone is often not sufficient to cause an acute TTP; additional factors may be
Q P Dou et al.
Cancer research, 53(7), 1493-1497 (1993-04-01)
Progression of cells into S phase is proposed to be determined by accumulation of a labile protein (the restriction point protein R; A. B. Pardee, Proc. Natl. Acad. Sci. USA, 71: 1286-1290, 1974). We report here that cyclin E and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service