G6751
α-Glycerophosphate Dehydrogenase from rabbit muscle
Type I, ammonium sulfate suspension, 100-300 units/mg protein
Synonym(s):
sn-Glycerol-3-phosphate Dehydrogenase from rabbit muscle, sn-Glycerol-3-phosphate:NAD+ 2-oxidoreductase
About This Item
Recommended Products
biological source
rabbit muscle
Quality Level
type
Type I
form
ammonium sulfate suspension
specific activity
100-300 units/mg protein
storage condition
(Tightly closed)
technique(s)
activity assay: suitable
color
white
foreign activity
Lactic dehydrogenase, pyruvate kinase, aldolase, and glyceraldehyde-3-phosphate dehydrogenase ≤0.01%
Triosephosphate isomerase ≤0.02%
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Research area: Cell Signaling
Application
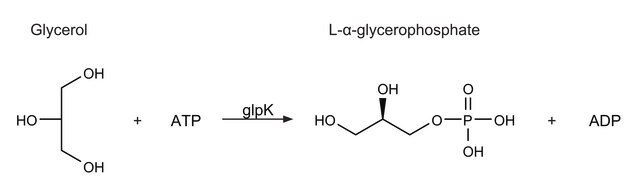
- in the reaction mixture to measure the glycerol kinase activity
- to demonstrate compartmentalized enzymatic reactions where NADH is involved
- in the reaction mixture assay for L-fuculose-1-phosphate aldolase
Biochem/physiol Actions
Unit Definition
Physical form
Preparation Note
Analysis Note
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Instructions for working with enzymes supplied as ammonium sulfate suspensions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service