All Photos(1)
About This Item
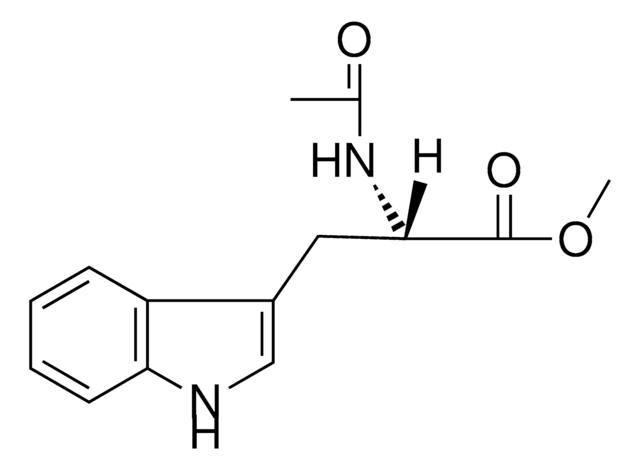
Empirical Formula (Hill Notation):
C13H15N3O2
CAS Number:
Molecular Weight:
245.28
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
N-Acetyl-L-tryptophanamide,
Assay
≥98%
Quality Level
form
powder
color
white to off-white
mp
194-196 °C (lit.)
application(s)
detection
storage temp.
−20°C
SMILES string
CC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O
InChI
1S/C13H15N3O2/c1-8(17)16-12(13(14)18)6-9-7-15-11-5-3-2-4-10(9)11/h2-5,7,12,15H,6H2,1H3,(H2,14,18)(H,16,17)/t12-/m0/s1
InChI key
HNGIZKAMDMBRKJ-LBPRGKRZSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
N-Acetyl-L-tryptophanamide (NATA) is an N-terminal and C-terminal blocked analogue of L-tryptophan. L-tryptophan, NATA and NATA-tyr molecules have intrinsic fluorescence which makes them useful in studies involving fluorescence and flurosence enhancement.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alexander V Fonin et al.
PloS one, 9(7), e103878-e103878 (2014-07-30)
Fluorescence is a proven tool in all fields of knowledge, including biology and medicine. A significant obstacle in its use is the nonlinearity of the dependence of the fluorescence intensity on fluorophore concentration that is caused by the so-called primary
Patrizia Cioni et al.
Biophysical journal, 82(6), 3246-3253 (2002-05-23)
The effects of heavy water (D(2)O) on internal dynamics of proteins were assessed by both the intrinsic phosphorescence lifetime of deeply buried Trp residues, which reports on the local structure about the triplet probe, and the bimolecular acrylamide phosphorescence quenching
Billie J Harvey et al.
The journal of physical chemistry. B, 111(10), 2610-2620 (2007-02-16)
Bovine beta-lactoglobulin A (BLGA) is a well characterized globular protein whose tertiary structure has been investigated in detail. BLGA undergoes a pH-dependent conformational change which X-ray data described as involving mostly the loop connecting strands E and F and the
A Buzády et al.
Biophysical chemistry, 88(1-3), 153-163 (2001-01-11)
The dielectric relaxation (DR) of human serum albumin (HSA) was studied by the method of phase-fluorometry. The protein environment of the single tryptophan in HSA shows a relatively low-speed DR of sub-ns characteristic time. This relaxation can be measured as
Jianhua Xu et al.
The journal of physical chemistry. B, 113(35), 12084-12089 (2009-08-28)
Time-resolved fluorescence decay profiles of N-acetyl-l-tryptophanamide (NATA) and tryptophan (Trp) dipeptides of the form Trp-X and X-Trp, where X is another aminoacyl residue, have been investigated using an ultraviolet upconversion spectrophoto fluorometer with time resolution better than 350 fs, together
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service