All Photos(1)
About This Item
Empirical Formula (Hill Notation):
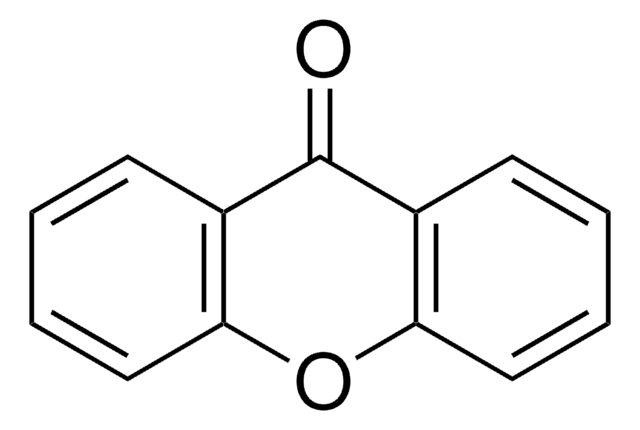
C13H8O2
CAS Number:
Molecular Weight:
196.20
Beilstein:
140443
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
bp
349-350 °C/730 mmHg (lit.)
mp
172-174 °C (lit.)
SMILES string
O=C1c2ccccc2Oc3ccccc13
InChI
1S/C13H8O2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1-8H
InChI key
JNELGWHKGNBSMD-UHFFFAOYSA-N
Gene Information
mouse ... Prkch(18755)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rajan Giri et al.
Bioorganic & medicinal chemistry, 18(4), 1456-1463 (2010-02-05)
A series of substituted xanthenes was synthesized and screened for activity using DU-145, MCF-7, and HeLa cancer cell growth inhibition assays. The most potent compound, 9 g ([N,N-diethyl]-9-hydroxy-9-(3-methoxyphenyl)-9H-xanthene-3-carboxamide), was found to inhibit cancer cell growth with IC(50) values ranging from
Christiane Müller et al.
Journal of the American Chemical Society, 133(41), 16689-16697 (2011-10-01)
Six 2-quinolones, which bear a terminal alkene linked by a three- or four-membered tether to carbon atom C4 of the quinolone, were synthesized and subjected to an intramolecular [2 + 2]-photocycloaddition. The reaction delivered the respective products in high yields
Michael A Schätzle et al.
Journal of the American Chemical Society, 134(36), 14742-14745 (2012-08-23)
Reduction of emodin by sodium dithionite resulted in the formation of two tautomeric forms of emodin hydroquinone. Subsequent conversion by the short-chain dehydrogenase/reductase (SDR) MdpC into the corresponding 3-hydroxy-3,4-dihydroanthracen-1(2H)-one implies that deoxygenation is the first step in monodictyphenone biosynthesis. Implications
Ping Wang et al.
Organic letters, 14(3), 902-905 (2012-01-26)
A concise and straightforward strategy to construct a xanthone skeleton via an intramolecular cross-dehydrogenative coupling (CDC) of 2-aryloxybenzaldehydes has been developed. The reaction proceeded smoothly without any need of preactivation of the aldehyde group. It can tolerate various functional groups
Asako Murata et al.
Bioorganic & medicinal chemistry letters, 23(1), 252-255 (2012-11-21)
In recent years, various biological processes have been found to be regulated by miRNA-mediated gene silencing. A small molecule that modulate the miRNA pathway will provide the biological tool for elucidating mechanisms of miRNA-mediated gene regulation, and can be the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service