P1781
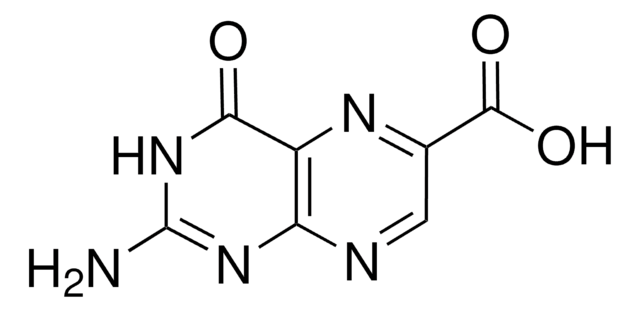
Pteroic acid
≥93%
Synonym(s):
4-{[(2-Amino-4-hydroxypteridin-6-yl)methyl]amino}benzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12N6O3
CAS Number:
Molecular Weight:
312.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥93%
form
powder
SMILES string
NC1=NC(=O)C2=NC(CNc3ccc(cc3)C(O)=O)=CNC2=N1
InChI
1S/C14H12N6O3/c15-14-19-11-10(12(21)20-14)18-9(6-17-11)5-16-8-3-1-7(2-4-8)13(22)23/h1-4,6,16H,5H2,(H,22,23)(H3,15,17,19,20,21)
InChI key
JOAQINSXLLMRCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Pteroic acid can be used as a reactant to synthesize:
- N-trifluoroacetyl pteroic acid by reacting with trifluoroacetic anhydride via acylation.
- bis-Decyl chain derivative of pteroic acid through photocleavage reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maciej Adamczyk et al.
Bioorganic & medicinal chemistry letters, 14(9), 2313-2317 (2004-04-15)
N(10)-Trifluoroacetylpteroic acid was conjugated to chemiluminescent N-sulfonylacridinium-9-carboxamide labels at the N(10) or 9-position carboxamide. Upon binding to folate binding protein the light output of the N(10) derivative (9) was quenched up to 62% upon triggering with basic peroxide, while the
Michael S Lee et al.
The journal of physical chemistry. B, 112(42), 13411-13417 (2008-09-30)
A comparative analysis is provided of the effect of different solvent models on the calculation of a potential of mean force (PMF) for determining the absolute binding affinity of the small molecule inhibitor pteroic acid bound to ricin toxin A-chain
C E Butterworth et al.
Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 181(1), 49-58 (1986-01-01)
"No-blood disease" is a severe, feed-related anemia of channel catfish. Pathological features in advanced cases include almost total absence of circulating red blood cells, hepatic fatty change, megaloblastic arrest of hematopoiesis, and intussusceptions of the small intestine. In view of
Cristina Müller et al.
European journal of nuclear medicine and molecular imaging, 33(9), 1007-1016 (2006-06-10)
The folate receptor (FR) is a valuable tumour marker, since it is frequently overexpressed on various cancer types. The purpose of the present study was to pre-clinically evaluate novel site-specifically modified (99m)Tc(CO)(3) folate (gamma-derivative 4, alpha-derivative 5) and pteroate (6)
Ratnesh Jain et al.
International journal of pharmaceutics, 420(1), 147-155 (2011-09-03)
Enhanced intracellular internalization of the anti-cancer active idarubicin (IDA) was achieved through appropriate surface modification of IDA loaded propyl starch nanoparticles. This was conducted by synthesizing pteroic acid modified polyvinyl alcohol (ptPVA) and employing this stabilizer for formulating the said
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service