337358
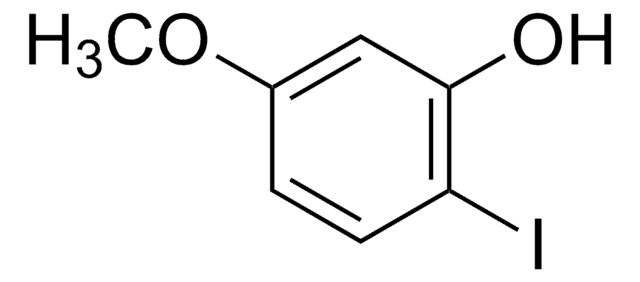
4-Iodo-2-methylphenol
97%
Synonym(s):
4-Iodo-o-cresol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H3(CH3)OH
CAS Number:
Molecular Weight:
234.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
105-110 °C/2 mmHg (lit.)
mp
67-68 °C (lit.)
functional group
iodo
SMILES string
Cc1cc(I)ccc1O
InChI
1S/C7H7IO/c1-5-4-6(8)2-3-7(5)9/h2-4,9H,1H3
InChI key
WSBDSSKIWDFOBQ-UHFFFAOYSA-N
General description
4-Iodo-2-methylphenol was prepared by direct iodination of 2-methylphenol in aqueous alcohol solvents by the action of a reagent prepared in situ from sodium hypochlorite and sodium iodide.
Application
4-Iodo-2-methylphenol was used as starting reagent in the synthesis of an agonist for the peroxisome proliferator-activated receptor δ (PPARδ) GW501516, a potential antiobesity drug.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A highly efficient synthesis of antiobestic ligand GW501516 for the peroxisome proliferator-activated receptor d through in situ protection of the phenol group by reaction with a Grignard reagent.
Ham J and Kang H.
Tetrahedron Letters, 46(39), 6683-6686 (2005)
An efficient and selective method for the preparation of iodophenols.
Edgar KJ and Falling SN.
The Journal of Organic Chemistry, 55(18), 5287-5291 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service