SML2860
Triptorelin acetate
≥98% (HPLC)
Synonym(s):
6-D-Tryptophan-Luteinizing Hormone-Releasing Factor acetate, [D-Trp6]-LH-RH acetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C64H82N18O13 · xC2H4O2
Molecular Weight:
1311.45 (free base basis)
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Biochem/physiol Actions
Triptorelin acetate is a synthetic decapeptide agonist analog of luteinizing hormone releasing hormone (LHRH). It is used clinically to treat prostate cancer and precocious puberty in boys and girls.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Successful desensitization to gonadotropin-releasing hormone analogue triptorelin acetate using a sustained-release depot preparation.
Pauline Chan Ng et al.
Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology, 29(6), 660-663 (2018-05-29)
Delphine Zenaty et al.
Hormone research in paediatrics, 86(3), 188-195 (2016-11-02)
To evaluate the efficacy and safety of a triptorelin pamoate (11.25 mg) 3-month formulation in the management of central precocious puberty (CPP) (TP Study) and to retrospectively compare it with a triptorelin acetate (11.25 mg) 3-month formulation (TA Study). We
Davide Meani et al.
Therapeutic advances in urology, 10(2), 51-63 (2018-02-13)
Androgen deprivation therapy (ADT) with luteinizing hormone-releasing hormone (LHRH) agonists is well established for the treatment of men with metastatic prostate cancer. As clear differences in efficacy, safety, or tolerability between the available LHRH agonists are lacking, the healthcare management
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service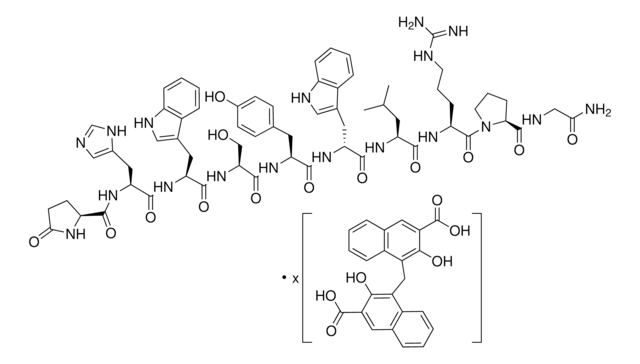


![[D-Trp6]-LH-RH ≥97% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/321/602/34cc2814-2f6f-4fc9-a266-835bfe27bcc5/640/34cc2814-2f6f-4fc9-a266-835bfe27bcc5.png)



