A1501
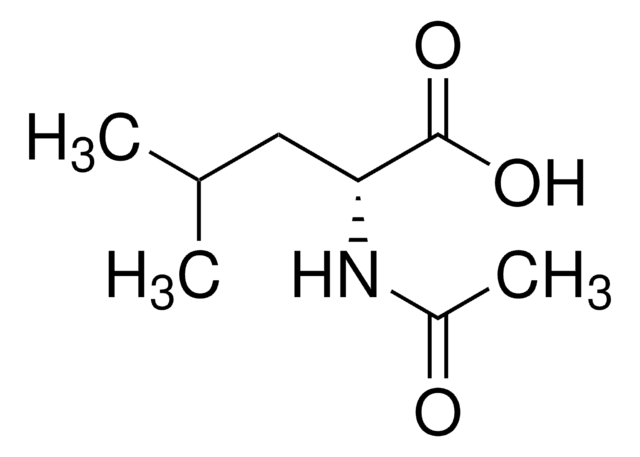
N-Acetyl-D-methionine
~99%, suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H13NO3S
CAS Number:
Molecular Weight:
191.25
Beilstein:
1725553
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
N-Acetyl-D-methionine, ~99%
Assay
~99%
Quality Level
form
powder or crystals
technique(s)
ligand binding assay: suitable
color
white
mp
102.3-103.6 °C
storage temp.
−20°C
SMILES string
CSCC[C@@H](NC(C)=O)C(O)=O
InChI
1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m1/s1
InChI key
XUYPXLNMDZIRQH-ZCFIWIBFSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Acetyl-D-methionine may be used as a substrate to identify, differentiate and characterized N-acylamino acid racemase(s) and N-acyl-D-amino acid amidohydrolase(s).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wen-Ching Wang et al.
Journal of molecular biology, 342(1), 155-169 (2004-08-18)
N-acylamino acid racemase (NAAAR) catalyzes the racemization of N-acylamino acids and can be used in concert with an aminoacylase to produce enantiopure alpha-amino acids, a process that has potential industrial applications. Here we have cloned and characterized an NAAAR homologue
Pei-Hsun Lin et al.
European journal of biochemistry, 269(19), 4868-4878 (2002-10-02)
An N-acyl-d-amino acid amidohydrolase (N-D-AAase) was identified in cell extracts of a strain, Iso1, isolated from an environment containing N-acetyl-d-methionine. The bacterium was classified as Variovorax paradoxus by phylogenetic analysis. The gene was cloned and sequenced. The gene consisted of
T Odajima et al.
Cell biochemistry and function, 16(2), 139-147 (1998-06-24)
Urate oxidase from Candida utilis, an enzyme containing an essential thiol, was examined for its sensitivity to lactoperoxidase, an oxidant present in breast milk. Upon exposure to a system composed of lactoperoxidase, hydrogen peroxide and bromide at moderately alkaline pH
Wen-Fang Ji et al.
The journal of physical chemistry. B, 111(2), 485-489 (2007-01-12)
The amino acid oxidation mechanism has been a research focus in recent years. Although various experimental techniques have been employed to address the problem, it is still a great challenge to identify the oxidation intermediates of amino acids. To explore
M J Wick et al.
Biochemical pharmacology, 37(7), 1225-1231 (1988-04-01)
Both N-hydroxy-2-acetamidofluorene (N-OH-AAF) and the heterocyclic analogue, 2-(N-hydroxyacetamido)carbazole (N-OH-AAC), were shown to be mechanism-based irreversible inhibitors (suicide inhibitors) of partially purified rat hepatic N-acetyltransferase (NAT) activity. Although N-OH-AAC exhibited an apparent first-order inactivation rate constant (ki) that was 7-fold lower
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service