A0760
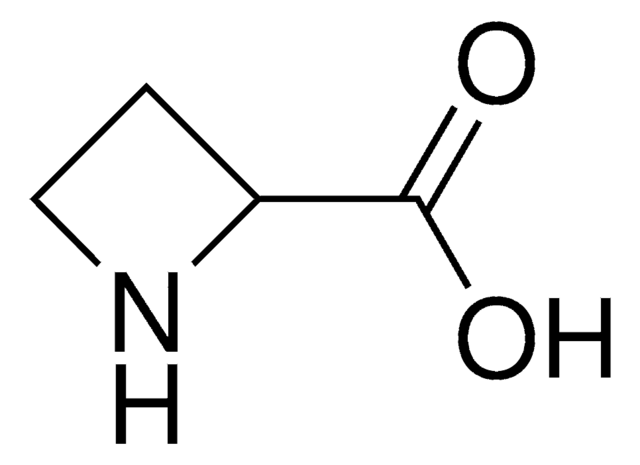
L-Azetidine-2-carboxylic acid
≥99%
Synonym(s):
(S)-Azetidine-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C4H7NO2
CAS Number:
Molecular Weight:
101.10
Beilstein:
80680
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
≥99%
form
powder
SMILES string
OC(=O)[C@@H]1CCN1
InChI
1S/C4H7NO2/c6-4(7)3-1-2-5-3/h3,5H,1-2H2,(H,6,7)/t3-/m0/s1
InChI key
IADUEWIQBXOCDZ-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
General description
L-Azetidine-2-carboxylic acid is a non-protein amino acid and teratogenic agent. It is toxic in nature.
Application
L-Azetidine-2-carboxylic acid has been used as a:
- collagen synthesis inhibitor
- protein folding antagonist
- as a standard in liquid chromatography-mass spectrometry
Biochem/physiol Actions
Azetidine-2-carboxylic acid (AZC) triggers protein aggregation or upregulates the expression of an aggregation-prone mutant protein, upon interference with nascent protein folding.
L-Azetidine-2-carboxylic acid is an inhibitor of collagen synthesis that is anti-angiogenic. It is a four-membered ring analog of L-proline that causes protein misconstruction when incorporated instead of proline.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Azetidine-2-carboxylic acid in garden beets (Beta vulgaris)
Rubenstein E, et al.
Phytochemistry, 67(9), 898-903 (2006)
Asmita Ghosh et al.
Cellular and molecular life sciences : CMLS, 76(8), 1605-1621 (2019-01-27)
The proteostasis network (PN) comprises a plethora of proteins that are dedicated to aid in protein folding and maintenance; some with overlapping functions. Despite this, there are multiple pathophysiological states associated with depletion of chaperones. This is counter-intuitive, assuming cells
The anticancer drug AUY922 generates a proteomics fingerprint that is highly conserved among structurally diverse Hsp90 inhibitors
Voruganti S, et al.
Journal of Proteome Research, 12(8), 3697-3706 (2013)
Ascorbic acid promotes the stemness of corneal epithelial stem/progenitor cells and accelerates epithelial wound healing in the cornea
Chen J, et al.
Stem Cells Translational Medicine, 6(5), 1356-1365 (2017)
Nadinath B Nillegoda et al.
Molecular biology of the cell, 21(13), 2102-2116 (2010-05-14)
Quality control systems facilitate polypeptide folding and degradation to maintain protein homeostasis. Molecular chaperones promote folding, whereas the ubiquitin/proteasome system mediates degradation. We show here that Saccharomyces cerevisiae Ubr1 and Ubr2 ubiquitin ligases promote degradation of unfolded or misfolded cytosolic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service