Y0001304
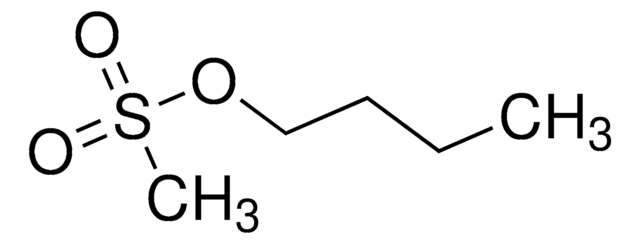
Butyl methanesulfonate
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
NSC 36060
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H12O3S
CAS Number:
Molecular Weight:
152.21
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
imatinib
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C5H12O3S/c1-3-4-5-8-9(2,6)7/h3-5H2,1-2H3
InChI key
LFLBHTZRLVHUQC-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Butyl methanesulfonate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Saffhill
Carcinogenesis, 5(5), 621-625 (1984-05-01)
The reaction of N-n-butyl-N-nitrosourea ( BNU ) and n-butyl methanesulphonate (BMS) with DNA has been investigated. In addition to the expected n- butylpurines formed on reaction with BNU some rearranged sec-butyl-adducts were also observed, indicating that a carbonium ion is
J C Ball et al.
Environmental and molecular mutagenesis, 13(2), 100-106 (1989-01-01)
This report describes experiments in which a chiral alkyl methanesulfonate was used to investigate possible mechanisms by which alkylating agents cause their mutagenic, cytotoxic, and clastogenic effects. Optically active enantiomers and the racemic mixture of 2-butyl methanesulfonate (2-BMS) were cytotoxic
A R Rao
International journal of cancer, 28(1), 105-110 (1981-07-15)
The influence of pregnant mare serum gonadotropin (PSGM) on the induction of ovarian tumors by carcinogen(DMBA) and/or mutagens (EMS and BMS) has been studied in Swiss albino mice. Priming of the ovaries of 6-week-old mice with PMSG (50 IU/mouse) 24
H Hoppe et al.
Mutation research, 250(1-2), 411-421 (1991-09-11)
Cells of the human lymphoblast line WI-L2 and its derivative TK-6 were synchronized by centrifugal elutriation and cell-cycle dependent mutation to 6TGR (HPRT) and OUAR (Na+, K+ ATPase) measured. Bromodeoxyuridine induced 6TGR and OUAR mutations within S phase while butylmethyl-sulfonate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service