All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H28S
CAS Number:
Molecular Weight:
252.46
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.488 (lit.)
bp
290 °C (lit.)
density
0.902 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCCCCCCCCCCCc1ccsc1
InChI
1S/C16H28S/c1-2-3-4-5-6-7-8-9-10-11-12-16-13-14-17-15-16/h13-15H,2-12H2,1H3
InChI key
RFKWIEFTBMACPZ-UHFFFAOYSA-N
Related Categories
General description
3-Dodecylthiophene (3-DT) is a conjugating monomer that can be used as an active layer on semiconductors. It has good electronic properties and can be used in the development of p-type semiconducting polymers. It is mainly used in the formation of poly(3-dodecylthiophene) (P3DT) through electrochemical polymerization. P3DT can further be utilized for a variety of organic electronic based applications.
Application
Conducting polymer precursor.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nuclear magnetic resonance studies on electrochemically prepared poly (3-dodecylthiophene)
Sato M and Morii H
Macromolecules, 24(5), 1196-1200 (1991)
Controlled charge transport by polymer blend dielectrics in top-gate organic field-effect transistors for low-voltage-operating complementary circuits
Baeg K, et al.
ACS Applied Materials & Interfaces, 4(11), 6176-6184 (2012)
Subthreshold characteristics of field effect transistors based on poly (3-dodecylthiophene) and an organic insulator
Scheinert S, et al.
Journal of Applied Physics, 92(1), 330-337 (2002)
Hiro Minamimoto et al.
Nanoscale, 13(3), 1784-1790 (2021-01-13)
Plasmon-induced chemical reactions triggered by near-infrared light illumination might enable efficient photo energy conversion. Here, electrochemical oxidative polymerization of a conductive polymer was conducted on plasmonic photoconversion electrodes. The absolute electrochemical potential of the generated holes was estimated from the
Journal of the American Chemical Society, 117, 233-233 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service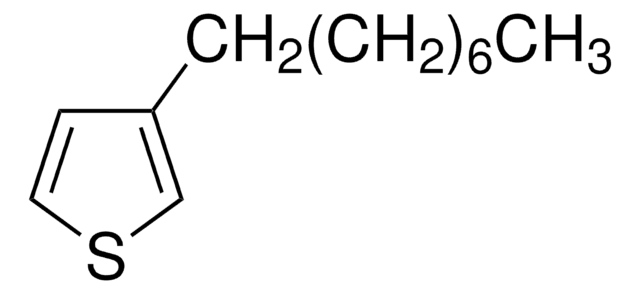

![(3aS,4R,5S,6aR)-(+)-Hexahydro-5-hydroxy-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one 98%](/deepweb/assets/sigmaaldrich/product/structures/235/039/9577fbe5-13a7-4410-b9c4-02727c3da799/640/9577fbe5-13a7-4410-b9c4-02727c3da799.png)