339822
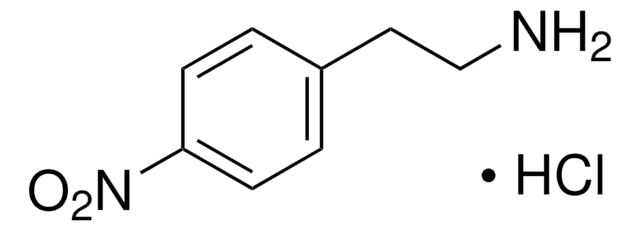
(S)-α-Methyl-4-nitrobenzylamine hydrochloride
97%
Synonym(s):
(S)-1-(4-Nitrophenyl)ethylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4CH(CH3)NH2 · HCl
CAS Number:
Molecular Weight:
202.64
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
optical activity
[α]25/D −6.5°, c = 1 in 0.05 M NaOH
mp
248-250 °C (lit.)
functional group
amine
nitro
SMILES string
Cl.C[C@H](N)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H10N2O2.ClH/c1-6(9)7-2-4-8(5-3-7)10(11)12;/h2-6H,9H2,1H3;1H/t6-;/m0./s1
InChI key
CZQQGVFHLSBEDV-RGMNGODLSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(S)-α-Methyl-4-nitrobenzylamine hydrochloride can be used as:
- A fluorescence-quenching guest in the determination of chiral recognition capabilities of binaphthocrown ether and polythiophene complex.
- A starting material in the synthesis of chiral cyclopalladated complexes with applications in the resolution of racemic compounds.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The first orthopalladation of a primary nitrobenzylamine. Synthesis of chiral cyclopalladated complexes derived from (S)-α-methyl-4-nitrobenzylamine
Vicente J, et al.
J. Chem. Soc., Dalton Trans., 47(15), 2535-2539 (1995)
Polymer-based supramolecular sensing and application to chiral photochemistry
Fukuhara G
Polymer Journal, 47(10), 649-655 (2015)
Gaku Fukuhara et al.
Chemical communications (Cambridge, England), 48(11), 1641-1643 (2011-12-15)
Chiral recognition abilities of the title host for (R)- and (S)-α-methyl-4-nitrobenzylamine were examined in the ground and excited states to give a relative affinity (K(R)/K(S)) of 2.16 by spectral titration and a relative rate constant (k(R)/k(S)) of 2.23 by fluorescence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service