All Photos(3)
About This Item
Linear Formula:
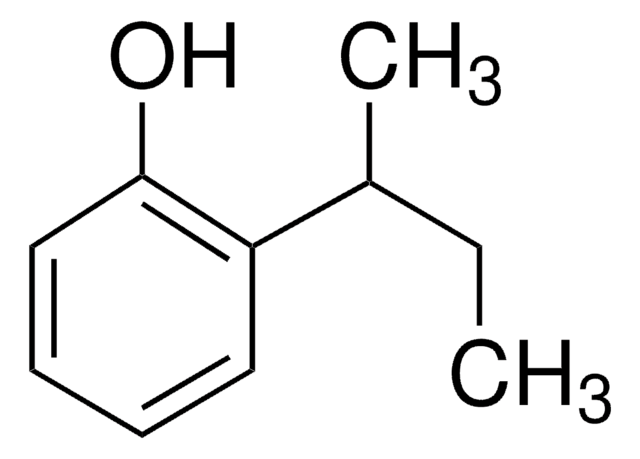
(CH3)2CHC6H4OH
CAS Number:
Molecular Weight:
136.19
Beilstein:
1363564
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
Assay
98%
form
crystals
bp
212-213 °C (lit.)
mp
59-61 °C (lit.)
SMILES string
CC(C)c1ccc(O)cc1
InChI
1S/C9H12O/c1-7(2)8-3-5-9(10)6-4-8/h3-7,10H,1-2H3
InChI key
YQUQWHNMBPIWGK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Sonochemical degradation of 4-Isopropylphenol has been studied.
Application
4-Isopropylphenol was used in the synthesis of vinyl compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - Pensky-Martens closed cup
Flash Point(C)
110 °C - Pensky-Martens closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
David J Hopper et al.
Applied and environmental microbiology, 69(6), 3650-3652 (2003-06-06)
4-ethylphenol methylenehydroxylase from Pseudomonas putida JD1 acts by dehydrogenation of its substrate to give a quinone methide, which is then hydrated to an alcohol. It was shown to be active with a range of 4-alkylphenols as substrates. 4-n-propylphenol, 4-n-butylphenol, chavicol
Mahdi Chiha et al.
Ultrasonics sonochemistry, 17(5), 773-782 (2010-04-15)
Sonochemical degradation of phenol (Ph), 4-isopropylphenol (4-IPP) and Rhodamine B (RhB) in aqueous solutions was investigated for a large range of initial concentrations in order to analyze the reaction kinetics. The initial rates of substrate degradation and H(2)O(2) formation as
D C Thompson et al.
Chemical research in toxicology, 8(1), 55-60 (1995-01-01)
The oxidative metabolism and toxicity of the para isomers of methylphenol (cresol), ethylphenol, and isopropylphenol were studied using male Sprague-Dawley rat liver microsomes and precision-cut liver slices. Reactive intermediates from each compound were trapped using radiolabeled glutathione and were detected
Frédéric L P Gabriel et al.
Chemistry & biodiversity, 4(9), 2123-2137 (2007-09-25)
Sphingobium xenophagum Bayram is capable of metabolizing 4-alkoxyphenols and endocrine disruptive alpha-quaternary 4-nonylphenols by an ipso-substitution mechanism that involves ring hydroxylation at the site of the substituent. Here, we show that Bayram's ipso-hydroxylating activity was able to transform also bisphenol
Ye Eun Park et al.
Nutrients, 12(1) (2020-01-23)
Probiotics can improve the intestinal environment by enhancing beneficial bacteria to potentially regulate lipid levels; however, the underlying mechanisms remain unclear. The aim of this study was to investigate the effect of Lactobacillus plantarum Q180 (LPQ180) on postprandial lipid metabolism
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service