A2103
Anti-Actin, N-terminal antibody produced in rabbit

~0.5 mg/mL, affinity isolated antibody, buffered aqueous solution
Synonym(s):
Actin Detection Antibody, Rabbit Anti-Actin
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
mol wt
antigen 42 kDa
species reactivity
frog, rat, mouse, chicken, human
packaging
antibody small pack of 25 μL
enhanced validation
independent
Learn more about Antibody Enhanced Validation
concentration
~0.5 mg/mL
technique(s)
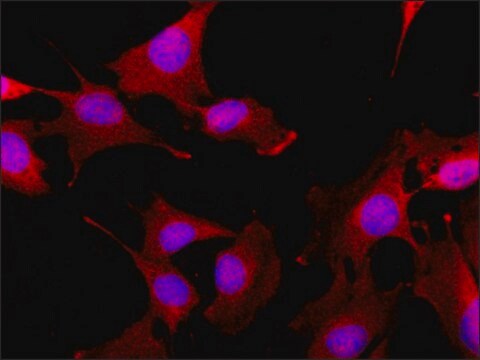
immunocytochemistry: 1-2 μg/mL using cultured chicken fibroblasts
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 2-4 μg/mL using sections of human appendix, mouse heart, and frog skeletal muscle
indirect immunofluorescence: suitable
western blot: 0.5-1 μg/mL using whole extract of the human epitheloid carcinoma HeLa cell line.
western blot: 2-4 ng/mL using whole extract of rat skeletal muscle
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... ACTA1(58) , ACTA2(59) , ACTB(60) , ACTC1(70) , ACTG1(71) , ACTG2(72)
mouse ... Acta1(11459) , Acta2(11475) , Actb(11461) , Actc1(11464) , Actg1(11465) , Actg2(11468)
rat ... Acta1(29437) , Acta2(81633) , Actb(81822) , Actc1(29275) , Actg1(287876) , Actg2(25365)
General description
Immunogen
Application
- immunoblotting
- quantitative chromatin immunoprecipitation (qChIP)
Biochem/physiol Actions
Physical form
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
recommended
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service