857225P
Avanti
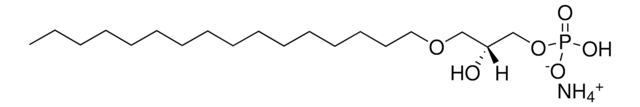
C16 Cyclic LPA
1-O-hexadecyl-sn-glycero-2,3-cyclic-phosphate (ammonium salt), powder
Synonym(s):
1-hexadecyl-sn-glycero-2,3-cyclic-phosphate (ammonium salt)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C19H42NO5P
CAS Number:
Molecular Weight:
395.51
UNSPSC Code:
51191904
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (857225P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 857225P
lipid type
phospholipids
cardiolipins
shipped in
dry ice
storage temp.
−20°C
SMILES string
O=P1([O-])O[C@H](COCCCCCCCCCCCCCCCC)CO1.[NH4+]
General description
Cyclic phosphatidic acid (cPA) is a naturally occurring analog of the growth factor-like phospholipid mediator, lysophosphatidic acid (LPA). The sn-2 hydroxy group of CPA forms a 5-membered ring with the sn-3 phosphate.
Application
C16 Cyclic LPA or 1-O-hexadecyl-sn-glycero-2,3-cyclic-phosphate (ammonium salt) has been used as an internal standard for the quantification of lysophosphatidic acid (LPA) in proton samples using reversed-phase ultra-performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS).
Biochem/physiol Actions
cPA affects numerous cellular functions, including anti-mitogenic regulation of the cell cycle, induction of stress fiber formation, inhibition of tumor cell invasion and metastasis, and regulation of differentiation and survival of neuronal cells. Interestingly, many of these cellular responses caused by cPA oppose those of LPA despite the activation of apparently overlapping receptor populations.
Packaging
5 mL Amber Glass Screw Cap Vial (857225P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Standardization and Quantification of Lysophosphatidic Acid Compounds by Normal-Phase and Reversed-Phase Chromatography-Tandem Mass Spectrometry
Moore JD, et al.
Lysophospholipid Receptors: Signaling and Biochemistry, 137-137 (2013)
Standardization and Quantification of Lysophosphatidic Acid Compounds by Normal-Phase and Reversed-Phase Chromatography-Tandem Mass Spectrometry
Moore JD, et al.
Lysophospholipid Receptors: Signaling and Biochemistry, 137-137 (2013)
Standardization and Quantification of Lysophosphatidic Acid Compounds by Normal-Phase and Reversed-Phase Chromatography-Tandem Mass Spectrometry
Moore JD, et al.
Lysophospholipid Receptors: Signaling and Biochemistry, 137-137 (2013)
K Murakami-Murofushi et al.
Cell structure and function, 18(5), 363-370 (1993-10-01)
The unique Physarum lysophosphatidic acid, PHYLPA, having a cyclopropane in the fatty acid moiety and a cyclic phosphate at C-2 and C-3 positions of the glycerol, inhibited proliferation of human fibroblast cells, TIG-3 and TIG-7, which were cultured in a
D J Fischer et al.
Molecular pharmacology, 54(6), 979-988 (1998-12-18)
Lysophosphatidic acid (LPA), plasmalogen-glycerophosphate (alkenyl-GP) and, cyclic-phosphatidic acid (cyclic-PA) are naturally occurring phospholipid growth factors (PLGFs). PLGFs elicit diverse biological effects via the activation of G protein-coupled receptors in a variety of cell types. In NIH3T3 fibroblasts, LPA and alkenyl-GP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service