KITALYSIS-RCM
KitAlysis™ High-Throughput Medium (5, 6, 7) Ring Closing Metathesis Reaction Screening Kit - Pack of 2
About This Item
Recommended Products
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
storage temp.
2-8°C
Application
Step-by-Step Guide for Ring Closing Metathesis/Cross Metathesis Reaction Screening Kit
Downloadable experimental excel file
Each kit contains 2 customizable screening sets that include the following components:
- 6x4 pre-weighed catalysts in glass microvials loaded with stir bars and topped with cap mat.
- 2 (2 mL) ampules each of of ClPh, PhMe, i-PrOAc, Me-THF, and TFA.
- 1 KitAlysis 24-well reaction block replacement film.
- 4 empty substrate stock solution reaction vials with stir bars.
- 1 internal standard.
Best when used with KitAlysis Benchtop Inertion Box and 24-well Reaction Block (sold separately).
Features and Benefits
- Designed and tested by synthetic chemists.
- Easily run 24-microscale reaction in parallel.
- 20 μmol reactions with 1 μmol catalyst (5 mol%), 0.2 M concentration, and optional TFA additive.
- Customizable and downloadable excel template for quick calculations and easy incorporation into electronic laboratory notebooks.
Legal Information
Kit Components Only
- SnapIT Ampule Opener, Regular, for 1-2ml, 5-10ml, 10-15ml ampoules 1 EA
Kit Components Also Available Separately
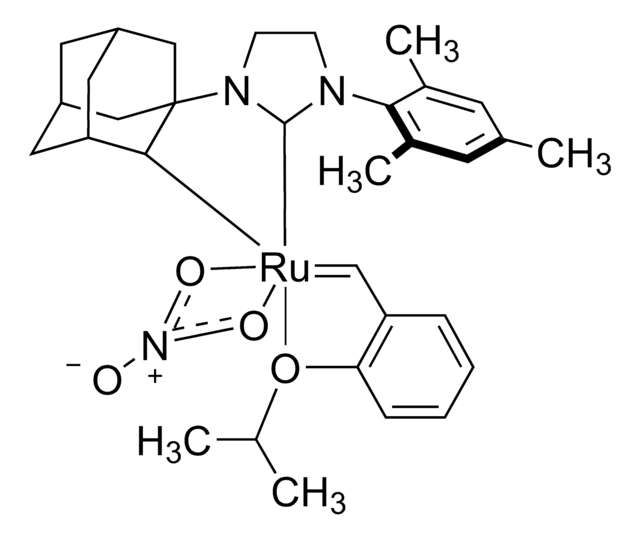
- 569747Grubbs Catalyst® M204, Umicore 3 µmolSDS
- 569755Hoveyda-Grubbs Catalyst® M720, Umicore, 97% 3 µmolSDS
- 579726Grubbs Catalyst® M102, Umicore, 97% 3 µmolSDS
- 682365Grubbs Catalyst® M207, Umicore 3 µmolSDS
- 682373Hoveyda-Grubbs Catalyst® M721, Umicore 3 µmolSDS
- 729345Hoveyda-Grubbs Catalyst® M722, Umicore 3 µmolSDS
- 900519Isopropyl acetate, anhydrous, ZerO2®, ≥99.6% 8 mLSDS
recommended
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 Inhalation - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service















![2,6-Diisopropylphenylimido-neophylidene[(S)-(−)-BIPHEN]molybdenum(VI) ringclosing metathesis catalyst, ≥95.0% (C)](/deepweb/assets/sigmaaldrich/product/structures/312/745/96ea840b-77a7-427a-9db5-fa08b3ffd45e/640/96ea840b-77a7-427a-9db5-fa08b3ffd45e.png)