All Photos(1)
About This Item
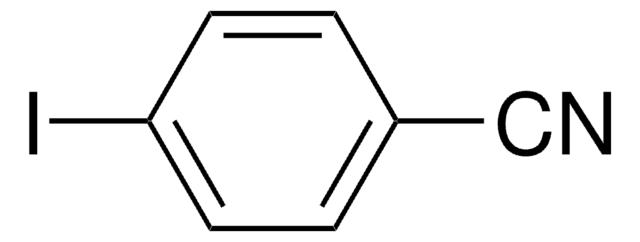
Linear Formula:
IC6H4CN
CAS Number:
Molecular Weight:
229.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
40-43 °C (lit.)
SMILES string
Ic1cccc(c1)C#N
InChI
1S/C7H4IN/c8-7-3-1-2-6(4-7)5-9/h1-4H
InChI key
BGARPMGQRREXLN-UHFFFAOYSA-N
General description
3-Iodobenzonitrile is a halogenated aromatic nitrile. Its standard (ρ° = 0.1MPa) molar enthalpy of formation was determined by combustion calorimetry.
Application
3-Iodobenzonitrile may be used as a starting reagent in the synthesis of tetrachloroisophthalo-[14C]-nitrile (TCIN). It may also be used in the preparation of:
- 1-(3-iodophenyl)-3-{2-[4-(trifluoromethyl)-1-piperidinyl]ethyl}-2-imidazolidinone
- piperidine derivative
- chiral amino acid anilide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of Chiral Amino Acid Anilides by Ligand-Free Copper-Catalyzed Selective N-Arylation of Amino Acid Amides
Dong J, et al.
Advanced Synthesis & Catalysis, 355(4), 692-696 (2013)
Synthesis of tetrachloroisophthalo-[14C]-nitrile.
Davies PE.
Journal of Labelled Compounds & Radiopharmaceuticals, 21(3), 285-292 (1984)
Dominic P Affron et al.
European journal of organic chemistry, 2016(1), 139-149 (2016-02-16)
Saturated heterocycles, such as THFs, pyrrolidines, piperidines and THPs, are essential components of many biologically active compounds. Examples of C-H functionalization on these important ring systems remain scarce, especially at unactivated positions. Here we report the development of conditions for
Idriss Bennacef et al.
Bioorganic & medicinal chemistry letters, 19(17), 5056-5059 (2009-07-29)
Compound 1 is a potent and selective antagonist of the dopamine D(3) receptor. With the aim of developing a carbon-11 labeled ligand for the dopamine D(3) receptor, 1 was selected as a potential PET probe. [(11)C]1 was obtained by palladium
Thermodynamic and aromaticity studies for the assessment of the halogen? cyano interactions on Iodobenzonitrile.
Rocha IM, et al.
The Journal of Chemical Thermodynamics, 65, 204-212 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service