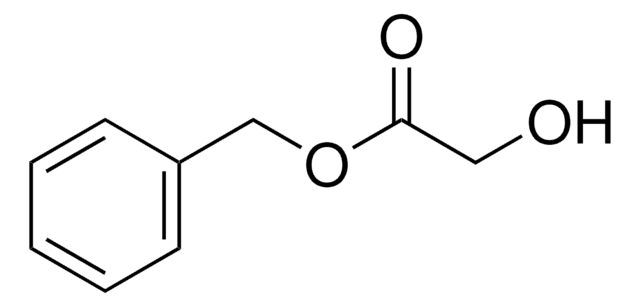
325260
Methyl glycolate
98%
Synonym(s):
Hydroxyacetic acid methyl ester, Methyl 2-hydroxyacetate, Methyl 2-hydroxyethanoate, Methyl hydroxyacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2CO2CH3
CAS Number:
Molecular Weight:
90.08
Beilstein:
1699571
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.12 (vs air)
vapor pressure
17 mmHg ( 52 °C)
Assay
98%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
149-151 °C (lit.)
density
1.167 g/mL at 25 °C (lit.)
functional group
ester
hydroxyl
SMILES string
COC(=O)CO
InChI
1S/C3H6O3/c1-6-3(5)2-4/h4H,2H2,1H3
InChI key
GSJFXBNYJCXDGI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Synthesis of methyl glycolate from the carbonylation of HCHO using heteropoly acids (HPAs) as catalysts, followed by esterification with methanol was reported. Size-selective vibrational spectroscopy of methyl glycolate clusters was reported.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly effective synthesis of methyl glycolate with heteropolyacids as catalysts.
Sun Y, et al.
Catalysis Communications, 10(5), 678-681 (2009)
Size-selective vibrational spectroscopy of methyl glycolate clusters: comparison with ragout-jet FTIR spectroscopy.
Farnik M, et al.
Physical Chemistry Chemical Physics, 6(19), 4614-4620 (2004)
Jong-Ryul Park et al.
Biointerphases, 16(1), 011001-011001 (2021-01-07)
Poly(2-alkyl-2-oxazoline) (PAOx) hydrogels are tailorable synthetic materials with demonstrated biomedical applications, thanks to their excellent biocompatibility and tunable properties. However, their use as injectable hydrogels is challenging as it requires invasive surgical procedures to insert the formed hydrogel into the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service