278394
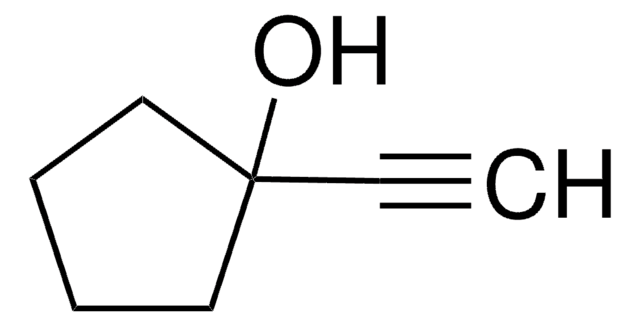
3,5-Dimethyl-1-hexyn-3-ol
98%
Synonym(s):
3,5-Dimethyl-1-hexyne-3-ol, 3,5-Dimethylhexyn-3-ol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2CHCH2C(CH3)(OH)C≡CH
CAS Number:
Molecular Weight:
126.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.34 (vs air)
Quality Level
vapor pressure
4.5 mmHg ( 20 °C)
Assay
98%
form
liquid
refractive index
n20/D 1.434 (lit.)
bp
150-151 °C (lit.)
density
0.859 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CC(C)CC(C)(O)C#C
InChI
1S/C8H14O/c1-5-8(4,9)6-7(2)3/h1,7,9H,6H2,2-4H3
InChI key
NECRQCBKTGZNMH-UHFFFAOYSA-N
General description
Bimolecular rate constant for the reaction of the hydroxyl radical (OH) with 3,5-dimethyl-1-hexyn-3-ol has been been measured using the relative rate technique. Reaction of CO2 with 3,5-dimethyl-1-hexyn-3-ol catalyzed by CuCl in different ionic liquids and solvents has been investigated.
Application
3,5-Dimethyl-1-hexyn-3-ol may be used to synthesize:
- 3,5-Dimethyl-1-phenyl-1-hexen-3-ol via one-pot palladium-mediated hydrostannylation/Stille cross-coupling.
- 3,5-dimethyl-1-hexyn-3-acetate via esterification with acetic anhydride in a neutral ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate).
- 3,5-Dimethyl-3-hydroxy-1-hexen-1-yl benzoate via anti-Markovnikov addition of benzoic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Neutral ionic liquid [BMIm] BF4 promoted highly selective esterification of tertiary alcohols by acetic anhydride.
Duan Z, et al.
J. Mol. Catal. A: Chem., 246(1-2), 70-75 (2006)
A New approach for the generation and reaction of organotin hydrides: the development of reactions catalytic in tin.
Terstiege I and Maleczka R E
The Journal of Organic Chemistry, 64(2), 342-343 (1999)
The hydroxyl radical reaction rate constant and products of 3, 5-dimethyl-1-hexyn-3-ol.
Wells JR.
International Journal of Chemical Kinetics, 36(10), 534-544 (2004)
Efficient Ruthenium?Catalysed Synthesis of 3?Hydroxy?1?propen?1?yl Benzoates: En Route to an Improved Isomerization of 2?Propyn?1?ols into α, β?Unsaturated Aldehydes.
Picquet M, et al.
European Journal of Organic Chemistry, 2000(13), 2361-2366 (2000)
Yanlong Gu et al.
The Journal of organic chemistry, 69(2), 391-394 (2004-01-17)
Reactions of propargylic alcohols with CO(2) in a [BMIm][PhSO(3)]/CuCl catalytic system to produce the corresponding alpha-methylene cyclic carbonates were conducted with high yields. Mild reaction conditions, enhanced rates, improved yields, and recyclable ionic liquid catalyst systems are the remarkable features
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service