145491
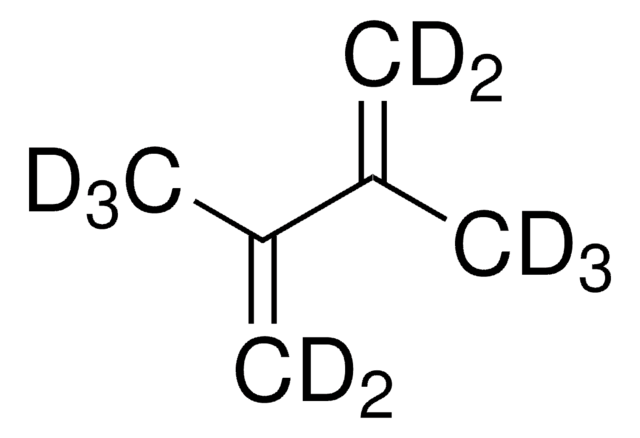
2,3-Dimethyl-1,3-butadiene
98%, contains 100 ppm BHT as stabilizer
Synonym(s):
2,3-Dimethylbuta-1,2-diene, 2,3-Dimethylenebutane, Biisopropenyl, Diisopropenyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=C(CH3)C(CH3)=CH2
CAS Number:
Molecular Weight:
82.14
Beilstein:
605285
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
269 mmHg ( 37.7 °C)
Quality Level
Assay
98%
form
liquid
contains
100 ppm BHT as stabilizer
refractive index
n20/D 1.438 (lit.)
bp
68-69 °C (lit.)
mp
−76 °C (lit.)
density
0.726 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=C)C(C)=C
InChI
1S/C6H10/c1-5(2)6(3)4/h1,3H2,2,4H3
InChI key
SDJHPPZKZZWAKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dimethyl-1,3-butadiene (DMBD) is a conjugated diene. It undergoes Diels Alder cycloaddition reaction with 2-thio-3-chloroacrylamides under thermal, catalytic and microwave conditions. It undergoes thermal [4+2] cycloaddition reaction with 3-acetyl-, 3-carbamoyl and 3-ethoxycarbonylcoumarins under solvent free conditions. DMBD participates in polymerization reactions in the presence of iron dichloride complexes based catalysts.
Application
2,3-Dimethyl-1,3-butadiene was used to investigate the reactions of 1,3-dienes with the Si(001) surface using scanning tunneling microscopy and fourier transform infrared spectroscopy.
It may be used in the following processes:
It may be used in the following processes:
- Preparation of 1,3,6-triene derivatives of corresponding 1-aryl-substituted 1,3-dienes by 1,4-hydrobutadienylation in the presence of cobalt catalyst.
- Synthesis of 6-aryl(hetaryl)-3,4-dimethyl-1-nitro-1-cyano-3-cyclohexenes by reacting with gem-cyanonitroethenes.
- As a halogen trap during the study of the photolysis reaction of dibromo adduct of 2,5-diphenyltellurophene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Peroxy radical kinetics resulting from the OH-initiated oxidation of 1,3-butadiene, 2,3-dimethyl-1,3-butadiene and isoprene.
Jenkin ME, et al.
Journal of Atmospheric Chemistry, 29(3), 267-298 (1998)
Cobalt-Catalyzed 1, 4-Hydrobutadienylation of 1-Aryl-1,3-dienes with 2,3-Dimethyl-1,3-butadiene.
Bohn MA, et al.
Angewandte Chemie (International Edition in English), 50(41), 9689-9693 (2011)
Polymerization of 1, 3-dienes with iron complexes based catalysts: Influence of the ligand on catalyst activity and stereospecificity.
Ricci G, et al.
J. Mol. Catal. A: Chem., 204, 287-293 (2003)
Aryl (hetaryl)-containing gem-cyanonitroethenes: Synthesis, structure, and reactions with 2,3-dimethyl-1,3-butadiene.
Baichurin RI, et al.
Russ. J. Gen. Chem., 85(8), 1845-1854 (2015)
Cycloaddition chemistry of 1, 3-dienes on the silicon (001) surface: Competition between [4+ 2] and [2+ 2] reactions.
Hovis JS, et al.
The Journal of Physical Chemistry B, 102(35), 6873-6879 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service