For information on the use of phenacyl bromide to derivatize carboxylic acids (e.g., fatty acids) for HPLC analysis, please see one or more of the following three references:1. K.J. Longmuir, et al., Determination of monoenoic fatty acid double bond position by permanganate-periodate oxidation followed by high-performance liquid chromatography of carboxylic acid phenacyl esters. Analytical Biochemistry, 167(2), 213-221 (1987).2. T. Hanis et al., Determination of fatty acids as phenacyl esters in rat adipose tissue and blood vessel walls by high-performance liquid chromatography. Journal of Chromatography, 452, 443-457 (1988). 3. A. Mehta et al., Rapid quantitation of free fatty acids in human plasma by high-performance liquid chromatography. Journal of Chromatography B Biomedical Science Applications, 719(1-2), 9-23 (1998).
77450
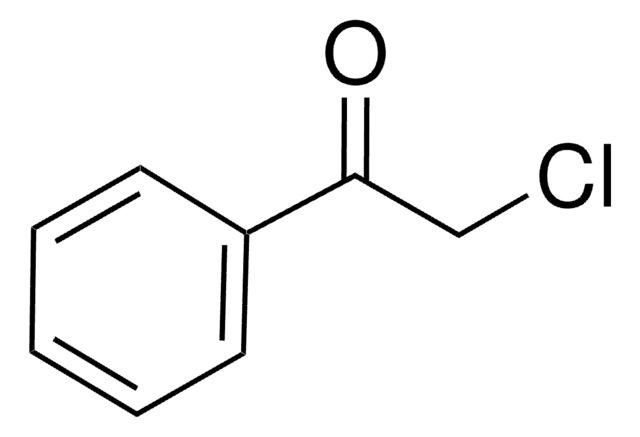
2-Bromoacetophenone
for GC derivatization, LiChropur™, ≥99.0%
Synonyme(s) :
ω-Bromoacetophenone, Phenacyl bromide
About This Item
Produits recommandés
Qualité
for GC derivatization
LiChropur™
Niveau de qualité
Essai
≥99.0% (GC)
≥99.0%
Forme
crystals
Pertinence de la réaction
reagent type: derivatization reagent
reaction type: Acylations
Technique(s)
gas chromatography (GC): suitable
pb
135 °C/18 mmHg (lit.)
Pf
48-51 °C (lit.)
49-50 °C
Température de stockage
2-8°C
Chaîne SMILES
BrCC(=O)c1ccccc1
InChI
1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
Clé InChI
LIGACIXOYTUXAW-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Skin Corr. 1B
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
235.4 °F - closed cup
Point d'éclair (°C)
113 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
-
Do you have references on the use of 2-Bromoacetophenone (also known as phenacyl bromide) Products 115835 and 77450 for making phenacyl esters of carboxylic acids for HPLC analysis?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique