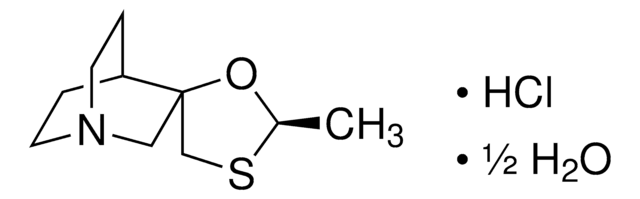
SML1244
Efinaconazole
≥98% (HPLC)
Synonym(s):
(αR,βR)-, α-(2,4-Difluorophenyl)-β-methyl-4-methylene-α-(1H-1,2,4-triazol-1-ylmethyl)-1-piperidineethanol, (2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol, KP-103
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
optical activity
[α]/D -85 to -95°, c = 1 in chloroform
color
white to beige
solubility
DMSO: 20 mg/mL, clear
storage temp.
−20°C
InChI
1S/C18H22F2N4O/c1-13-5-7-23(8-6-13)14(2)18(25,10-24-12-21-11-22-24)16-4-3-15(19)9-17(16)20/h3-4,9,11-12,14,25H,1,5-8,10H2,2H3/t14-,18-/m1/s1
InChI key
NFEZZTICAUWDHU-RDTXWAMCSA-N
1 of 4
This Item | Z694398 | Z687820 | Z694371 |
|---|---|---|---|
| suitability suitable for (multiple organ co-culture) | suitability suitable for (multiple organ co-culture) | suitability suitable for (multiple organ co-culture) | suitability suitable for (multiple organ co-culture) |
| material colorless wells, polystyrene | material clear polystyrene , clear wells | material colorless wells, polystyrene | material clear wells, polystyrene |
| sterility sterile | sterility sterile | sterility sterile | sterility sterile |
| packaging pack of 1 ea | packaging - | packaging pack of 5 ea | packaging - |
| manufacturer/tradename Advanced Pharmaceutical Sciences 71011 | manufacturer/tradename Advanced Pharmaceutical Sciences 71044 | manufacturer/tradename Advanced Pharmaceutical Sciences 71010 | manufacturer/tradename Advanced Pharmaceutical Sciences 71043 |
Application
- a topical anti-onychomycosis drug to determine its effects on Trichophyton rubrum and Trichophyton interdigitale[1]
- as an anti-fungal agent to study its permeability into the nail lysates[2]
- as an anti-fungal agent to study its effects on Candida africana and Candida dubliniensis[3]
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service