SML0762
Enzastaurin
≥98% (HPLC)
Synonym(s):
3-(1-Methyl-1H-indol-3-yl)-4-[1-[1-(2-pyridinylmethyl)-4-piperidinyl]-1H-indol-3-yl]-1H-pyrrole-2,5-dione, D04014, LY-317615, LY317615
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
color
, light orange to dark orange-red
solubility
DMSO: 10 mg/mL, clear (warmed)
storage temp.
−20°C
InChI
1S/C32H29N5O2/c1-35-19-25(23-9-2-4-11-27(23)35)29-30(32(39)34-31(29)38)26-20-37(28-12-5-3-10-24(26)28)22-13-16-36(17-14-22)18-21-8-6-7-15-33-21/h2-12,15,19-20,22H,13-14,16-18H2,1H3,(H,34,38,39)
InChI key
AXRCEOKUDYDWLF-UHFFFAOYSA-N
Application
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Glycogen synthase kinase 3 (GSK-3) is a highly conserved family of serine/threonine kinases for over 100 proteins in many pathways. See a list of accepted GSK-3 modulators and related products.
Glycogen synthase kinase 3 (GSK-3) is a highly conserved family of serine/threonine kinases for over 100 proteins in many pathways. See a list of accepted GSK-3 modulators and related products.
Glycogen synthase kinase 3 (GSK-3) is a highly conserved family of serine/threonine kinases for over 100 proteins in many pathways. See a list of accepted GSK-3 modulators and related products.
Glycogen synthase kinase 3 (GSK-3) is a highly conserved family of serine/threonine kinases for over 100 proteins in many pathways. See a list of accepted GSK-3 modulators and related products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service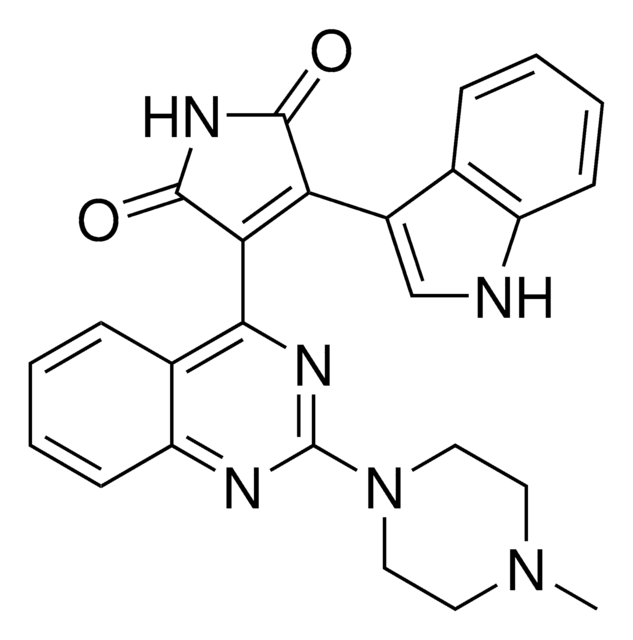
![2-Chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/378/828/9834c930-f37e-4730-8e9c-00a587e5421d/640/9834c930-f37e-4730-8e9c-00a587e5421d.png)