H107
(2-Hydroxypropyl)-β-cyclodextrin
powder
Synonym(s):
2-hydroxypropylether, Beta-cyclodextrin
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥98% (TLC)
form
powder
mol wt
estimated mol wt ~1396 Da
solubility
H2O: soluble
storage temp.
room temp
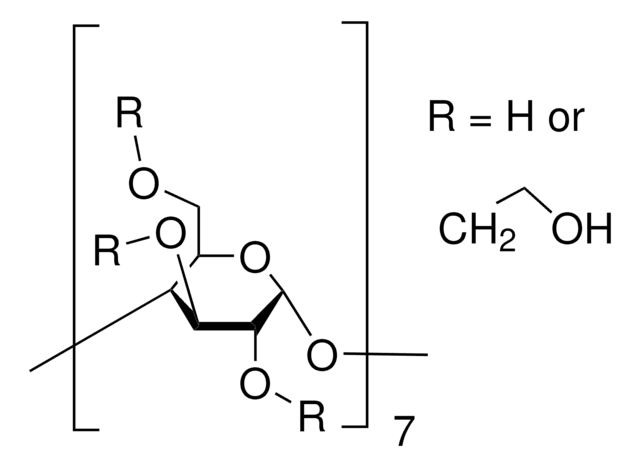
SMILES string
CC(O)COCC1OC2OC3C(COCC(C)O)OC(OC4C(COCC(C)O)OC(OC5C(COCC(C)O)OC(OC6C(COCC(C)O)OC(OC7C(COCC(C)O)OC(OC8C(COCC(C)O)OC(OC1C(OCC(C)O)C2OCC(C)O)C(OCC(C)O)C8OCC(C)O)C(OCC(C)O)C7OCC(C)O)C(OCC(C)O)C6OCC(C)O)C(OCC(C)O)C5OCC(C)O)C(OCC(C)O)C4OCC(C)O)C(OCC(C)O)C3OCC(C)O
InChI
1S/C63H112O42/c1-22(64)8-85-15-29-50-36(71)43(78)57(92-29)100-51-30(16-86-9-23(2)65)94-59(45(80)38(51)73)102-53-32(18-88-11-25(4)67)96-61(47(82)40(53)75)104-55-34(20-90-13-27(6)69)98-63(49(84)42(55)77)105-56-35(21-91-14-28(7)70)97-62(48(83)41(56)76)103-54-33(19-89-12-26(5)68)95-60(46(81)39(54)74)101-52-31(17-87-10-24(3)66)93-58(99-50)44(79)37(52)72/h22-84H,8-21H2,1-7H3/t22?,23?,24?,25?,26?,27?,28?,29-,30-,31?,32?,33?,34?,35?,36?,37-,38?,39-,40+,41+,42+,43?,44+,45?,46+,47+,48+,49+,50+,51+,52-,53-,54-,55-,56-,57+,58-,59+,60-,61-,62-,63-/m1/s1
InChI key
ODLHGICHYURWBS-RYJYQAAZSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The solubility of natural cyclodextrins is very poor. In the late 1960′s, it was discovered that chemical substitutions at the 2, 3, and 6 hydroxyl sites would greatly increase solubility. Most chemically modified cyclodextrins are able to achieve a 50% (w/v) concentration in water.
Cavity size is the major determinant as to which cyclodextrin is used in complexation. The cavity diameter of β-cyclodextrins or β-glucopyranose unit compounds is well-suited for use with molecules the size of hormones, vitamins and many compounds frequently used in tissue and cell culture applications. For this reason, ß-cyclodextrin is most commonly used as a complexing agent.
Application
- to investigate its effects on cholesterol homeostasis
- for the kindling procedure in mice and hippocampal electrographic analysis
- to improve poor prepulse inhibition (PPI) in mice
comparable product
replaced by
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cyclodextrin solubilizes fat-soluble vitamins and hormones, enhancing their solubility in water for cell culture applications.
Cyclodextrin solubilizes fat-soluble vitamins and hormones, enhancing their solubility in water for cell culture applications.
Cyclodextrin solubilizes fat-soluble vitamins and hormones, enhancing their solubility in water for cell culture applications.
Cyclodextrin solubilizes fat-soluble vitamins and hormones, enhancing their solubility in water for cell culture applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service