E6910
Pioglitazone hydrochloride
≥98% (HPLC), powder, hepatic gluconeogenesis blocker
Synonym(s):
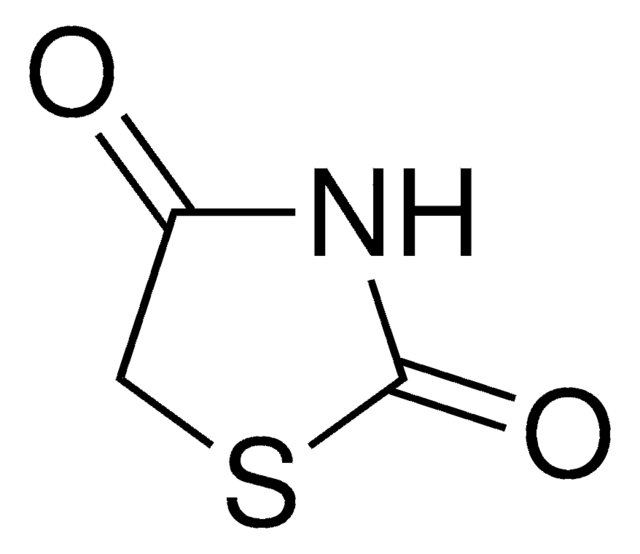
5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione monohydrochloride
About This Item
Recommended Products
product name
Pioglitazone hydrochloride, ≥98% (HPLC)
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to off-white
solubility
DMSO: ≥10 mg/mL
originator
Takeda
storage temp.
room temp
SMILES string
Cl.CCc1ccc(CCOc2ccc(CC3SC(=O)NC3=O)cc2)nc1
InChI
1S/C19H20N2O3S.ClH/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17;/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23);1H
InChI key
GHUUBYQTCDQWRA-UHFFFAOYSA-N
Gene Information
human ... PPARG(5468)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to administer to mice model and treated the hepatoma cell line to study its effect on regulating insulin-degrading enzyme (IDE) in diet-induced obese (DIO) C57BL/6 mice
- in drug preparation to analyze its effects on shortening and calcium transport in ventricular myocytes from the Goto-Kakizaki (GK) type 2 diabetic rat
- to treat HepG2 cells with peroxisome proliferator-activated receptor γ (PPARγ) agonists to examine its effect on TOMM40-, APOE- and APOC1-mRNA levels
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service