37520
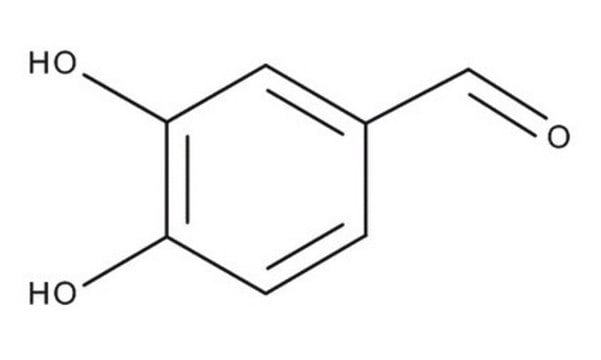
3,4-Dihydroxybenzaldehyde
purum, ≥97.0% (HPLC)
Synonym(s):
Protocatechualdehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein:
774381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (HPLC)
form
powder
mp
150-155 °C
150-157 °C (lit.)
SMILES string
Oc1ccc(C=O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)7(10)3-5/h1-4,9-10H
InChI key
IBGBGRVKPALMCQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3,4-Dihydroxybenzaldehyde has been recognized as one of the antifungal compound extracted from the outer skin of green Cavendish bananas. It can be synthesized from catechol via Fries rearrangement.
3,4-Dihydroxybenzaldehyde is reported as bioactive compound which inhibits the H2O2-induced apoptosis of granulosa cells. Oxidation of 3,4-dihydroxybenzaldehyde on glassy carbon electrodes is reported to afford stable redox-active electropolymerized films containing a quinone moity.
Application
3,4-Dihydroxybenzaldehyde (Protocatechualdehyde) may be employed as starting reagent for the synthesis of 4-vinylbenzocrown ether.
3,4-Dihydroxybenzaldehyde may be used for the surface modification of nanocrystalline TiO2 particles. Electrodeposited layer of 3,4-dihydroxybenzaldehyde may be used as effective redox mediator during oxidation of NADH at graphene. It may be used in the preparation of new diSchiff base ligands, which forms di-, tri- and tetranuclear Co(II) and Cu(II) complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 4'-vinylbenzocrown ethers.
Smid J, et al.
Organic Prep. and Proc. Int., 8(4), 193-196 (1976)
Electrocatalysis of NADH oxidation with electropolymerized films of 3, 4-dihydroxybenzaldehyde.
Pariente F, et al.
Analytical Chemistry, 66(23), 4337-4344 (1994)
Tatjana D Savić et al.
Physical chemistry chemical physics : PCCP, 16(38), 20796-20805 (2014-08-29)
The surface modification of nanocrystalline TiO2 particles (45 Å) with catecholate-type ligands having different electron donating/electron withdrawing substituent groups, specifically 3-methylcatechol, 4-methylcatechol, 3-methoxycatechol, 3,4-dihydroxybenzaldehyde and 4-nitrocatechol, was found to alter the optical properties of nanoparticles in a similar way to
3, 4-dihydroxybenzaldehyde, a fungistatic substance from green Cavendish bananas.
Mulvena D, et al.
Phytochemistry, 8(2), 393-395 (1969)
Nicole L Ritzert et al.
Faraday discussions, 172, 27-45 (2014-11-27)
Over the past decade, there has been a great deal of interest in graphene with regards to its electrochemical behavior. Previous studies have focused on understanding fundamental processes such as charge transfer and molecular transport at the graphene-electrolyte interface as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service