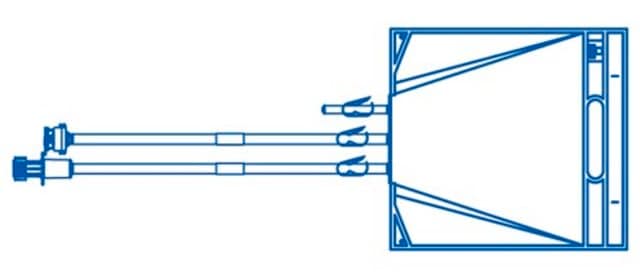
NAU0010L30EP5
Mobius® Silver 2D Assembly End Ported Bag
10L Standard PureFlex™
Synonym(s):
Mobius®, Silver, 10 L Standard PureFlex™ End Ported Bag
About This Item
Recommended Products
material
PureFlex™ film (with hanger and support rod)
Quality Level
product line
EMPROVE® Single Use
Mobius®
parameter
10 L sample volume
L
62.1 cm (24.4 in.)
W
30.0 cm (11.8 in.)
fitting
female Luer outlet fitting (with cap)
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | NAU0020L30EP5 | SAU0001L20EP10 | NAU0050L30EP5 |
|---|---|---|---|
| fitting female Luer outlet fitting (with cap) | fitting female Luer outlet fitting (with cap) | fitting female Luer outlet fitting (with cap) | fitting female Luer outlet fitting (with cap) |
| material PureFlex™ film (with hanger and support rod) | material PureFlex™ film (with hanger and support rod) | material PureFlex™ film (with hanger) | material PureFlex™ film (with hanger and support rod) |
| parameter 10 L sample volume | parameter 20 L sample volume | parameter 1 L sample volume | parameter 50 L sample volume |
| L 62.1 cm (24.4 in.) | L 65.4 cm (25.7 in.) | L 30.8 cm (12.1 in.) | L 82.6 cm (32.5 in.) |
| W 30.0 cm (11.8 in.) | W 43.1 cm (16.9 in.) | W 15.0 cm (5.9 in.) | W 58.4 cm (23.0 in.) |
Components
C-Flex 374 tubing, 3/8 in. ID x 5/8 in. OD, 18 in. long
Platinum-cured Silicone, 1/4 in. ID x 1/2 in. OD, 18 in. long
Pinch clamp
Legal Information
Not finding the right product?
Try our Product Selector Tool.
What is the Emprove® Program?
The Emprove® Program is a system providing comprehensive and thorough documentation of our filters and single-use components, pharma raw materials, and starting materials. Four document types are included with an Emprove® Program subscription:
Free of charge - Sign in to download
Free of charge - Sign in to download
Free of charge - Sign in to download
Free of charge - Sign in to download
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service