W238503
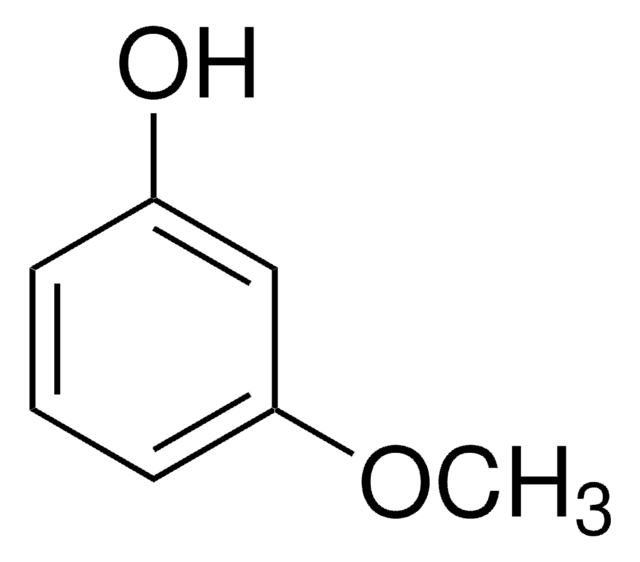
1,3-Dimethoxybenzene
≥98%, FG
Synonym(s):
Dimethylresorcinol, Resorcinol dimethyl ether
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
Assay
≥98%
refractive index
n20/D 1.524 (lit.)
bp
85-87 °C/7 mmHg (lit.)
density
1.055 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
medicinal; chemical; cooling; sweet
SMILES string
COc1cccc(OC)c1
InChI
1S/C8H10O2/c1-9-7-4-3-5-8(6-7)10-2/h3-6H,1-2H3
InChI key
DPZNOMCNRMUKPS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Homogeneous Gold Catalysis Using Complexes Recovered from Waste Electronic Equipment.: This research utilizes 1,3-Dimethoxybenzene in catalytic processes involving gold complexes reclaimed from electronic waste, presenting a sustainable approach in catalytic chemistry and highlighting the reuse of precious metals in industrial applications (McCarthy et al., 2022).
- Sequential Ir/Cu-Mediated Method for the Meta-Selective C-H Radiofluorination of (Hetero)Arenes.: Demonstrates a technique for the meta-selective C-H radiofluorination involving 1,3-Dimethoxybenzene, offering a novel pathway for the synthesis of radio-labelled compounds used in PET imaging, which is crucial for medical diagnostics and research (Wright et al., 2021).
- Synthesis and antioxidant activities of phenol derivatives from 1,6-bis(dimethoxyphenyl)hexane-1,6-dione.: Discusses the synthesis of new antioxidant compounds derived from 1,3-Dimethoxybenzene, underlining its role in developing therapeutic agents that could mitigate oxidative stress-related diseases (Artunc et al., 2020).
- One-Step Synthesis of C(2v)-Symmetric Resorcin[4]arene Tetraethers.: This paper details a one-step synthesis method using 1,3-Dimethoxybenzene to create novel macrocyclic molecules, potentially useful in host-guest chemistry and molecular recognition, which are key areas in supramolecular chemistry and nanotechnology (Smith et al., 2020).
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service