D223204
Duroquinone
97%
Synonym(s):
2,3,5,6-Tetramethyl-1,4-benzoquinone, Tetramethyl-p-benzoquinone
Select a Size
Select a Size
About This Item
Recommended Products
Assay
97%
form
powder
mp
110-112 °C (lit.)
SMILES string
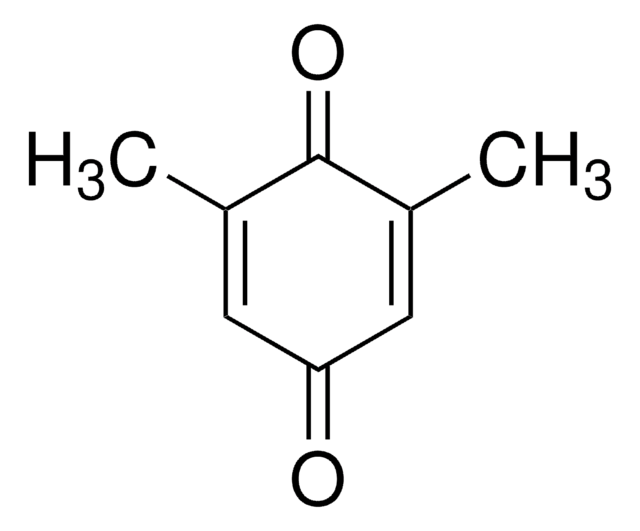
CC1=C(C)C(=O)C(C)=C(C)C1=O
InChI
1S/C10H12O2/c1-5-6(2)10(12)8(4)7(3)9(5)11/h1-4H3
InChI key
WAMKWBHYPYBEJY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Role of Duroquinone in Photosynthetic Research: A study on purple bacterial photosynthetic reaction centers highlighted Duroquinone′s role when incorporated into the QA binding site, impacting the isotope edited FTIR difference spectra, crucial for understanding energy conversion processes in photosynthesis (Zhao et al., 2013).
- Duroquinone as a Molecular Ion Source: Research demonstrated the generation of molecular negative ions by Duroquinone, showcasing its potential in mass spectrometry applications for studying molecular ionization and longevity (Khvostenko et al., 2012).
- Duroquinone in Biochemical Sensors: Investigation into the optimization of gold electrode surfaces with photosystem II monolayers revealed Duroquinone′s critical role in enhancing sensor responses, applicable in biochemical sensor technology (Maly et al., 2005).
- Cytotoxicity of Duroquinone Congeners: A comparative study of 14 p-benzoquinone congeners, including Duroquinone, used quantitative structure-toxicity relationships to evaluate cytotoxicity in rat hepatocytes and PC12 cells, relevant for safety assessments in chemical manufacturing (Siraki et al., 2004).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Need A Sample COA?
This is a sample Certificate of Analysis (COA) and may not represent a recently manufactured lot of this specific product.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service