D182001
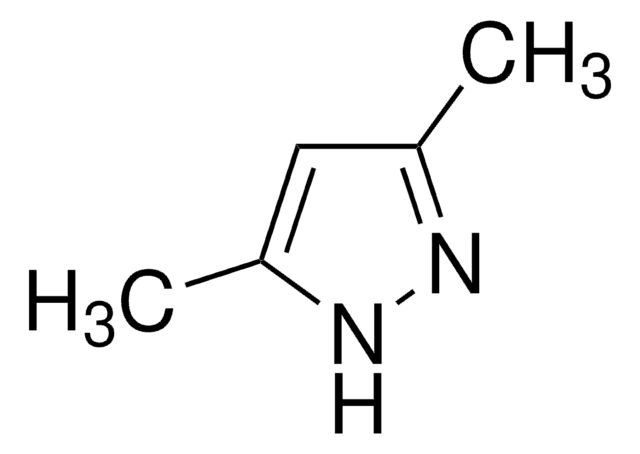
3,5-Dimethylpyrazole
99%
Synonym(s):
3,5-Dimethyl-1H-pyrazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8N2
CAS Number:
Molecular Weight:
96.13
Beilstein:
106325
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39160503
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
bp
218 °C (lit.)
mp
105-108 °C (lit.)
SMILES string
Cc1cc(C)[nH]n1
InChI
1S/C5H8N2/c1-4-3-5(2)7-6-4/h3H,1-2H3,(H,6,7)
InChI key
SDXAWLJRERMRKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Common reagent for the preparation of pyrazolato ligated complexes. Also used to prepare N-1-substituted derivatives having antibacterial activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - STOT RE 2
Target Organs
Liver
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of Organometallic Chemistry, 443, 221-221 (1993)
J. Chem. Soc., Dalton Trans., 3625-3625 (1993)
J. Chem. Soc. Pak., 14, 125-125 (1992)
Rupam Sarma et al.
Dalton transactions (Cambridge, England : 2003), (36)(36), 7428-7436 (2009-09-04)
The reactions of 3,5-dimethylpyrazole with zinc(II)acetate dihydrate and varieties of aromatic carboxylic acids led to formation of mono-nuclear zinc complexes of composition [Zn(HDMP)2(RCO2)2] (R = C6H5, p-CH3-C6H4, p-NO2-C6H4 etc. HDMP = 3,5-dimethylpyrazole) in methanol, whereas the same reactants in dimethylformamide
A Del Roso et al.
Acta biologica Hungarica, 42(1-3), 87-99 (1991-01-01)
In this study, we explored the changes in the rate of protein degradation in liver cells in vivo, using a method based on the physiological stimulation of liver autophagy. Male albino rats 1, 2, 6, 12 and 24 months old
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service